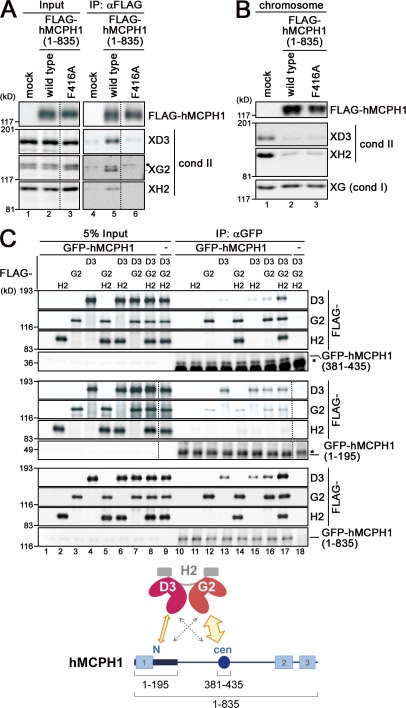
Figure 6.
hMCPH1 physically interacts with condensin II through its N-terminal and central domains. (A) A reticulocyte lysate containing no hMCPH1 (mock) or FLAG-tagged hMCPH1 was mixed with 10 vol metaphase egg extracts and incubated for 60 min. Anti-FLAG beads were then added into the mixtures, and bound fractions and aliquots of the mixtures were analyzed by immunoblotting. Different levels of contrast adjustment were performed between the input and immunoprecipitation (IP) blots. The FLAG data are derived from a different membrane than the condensin (cond) II data. Nonspecific bands that cross reacted with anti–XCAP-G2 (XG2) are indicated by the asterisk. (B) Sperm chromatin was incubated with the mixtures as described in A for 120 min, and chromosome fractions were isolated and analyzed by immunoblotting. (C) FLAG-tagged non-SMC subunits of human condensin II (CAP-D3, -G2, and -H2) were translated individually in reticulocyte lysates and mixed in different combinations as indicated. Three different versions of GFP-tagged hMCPH1 were produced in different reactions, incubated with the aforementioned mixtures, and subjected to immunoprecipitation with anti-GFP. The immunoprecipitates and 5% of input fractions were analyzed by immunoblotting with anti-FLAG and anti-GFP. FLAG data are derived from a different membrane than the GFP data for the top and middle datasets. The asterisks indicate nonspecific bands. The major binding partners of the central (cen; amino acids 381–435) and N-terminal (N; amino acids 1–195) domains of hMCPH1 are CAP-G2 and -D3, respectively (cartoon). The dotted lines indicate where intervening lanes were removed for presentation purposes. XH2, XCAP-H2.