Abstract
Multiple diameter single fiber reflectance (MDSFR) measurements of turbid media can be used to determine the reduced scattering coefficient (μ′s) and a parameter that characterizes the phase function (γ). The MDSFR method utilizes a semi-empirical model that expresses the collected single fiber reflectance intensity as a function of fiber diameter (dfiber), μ′s, and γ. This study investigated the sensitivity of the MDSFR estimates of μ′s and γ to the choice of fiber diameters and spectral information incorporated into the fitting procedure. The fit algorithm was tested using Monte Carlo simulations of single fiber reflectance intensities that investigated biologically relevant ranges of scattering properties (μ′s ∈ [0.4 – 4]mm−1) and phase functions (γ ∈ [1.4 – 1.9]) and for multiple fiber diameters (dfiber ∈ [0.2 – 1.5] mm). MDSFR analysis yielded accurate estimates of μ′s and γ over the wide range of scattering combinations; parameter accuracy was shown to be sensitive to the range of fiber diameters included in the analysis, but not to the number of intermediate fibers. Moreover, accurate parameter estimates were obtained without a priori knowledge about the spectral shape of γ. Observations were used to develop heuristic guidelines for the design of clinically applicable MDSFR probes.
OCIS codes: (170.1470) Blood or tissue constituent monitoring; (170.3660) Light propagation in tissues; (170.6510) Spectroscopy, tissue diagnostics; (290.7050) Turbid media; (300.6540) Spectroscopy, ultraviolet; (300.6550) Spectroscopy, visible
1. Introduction
Reflectance spectroscopy is a non-invasive method that is widely used to measure tissue optical properties. Such information can characterize vascular physiology and tissue ultrastructure, factors that may have diagnostic value [1, 2, 3]. Tissue optical properties are characterized by the absorption coefficient (μa) and the scattering coefficient (μs), which are the inverse of the mean free paths between absorption and scattering events, respectively, as well as the scattering phase function (PF), which describes the angular distribution of scattering events. Light transport at large distances from the light source (in biological tissues this is generally more than 2 mm) can be described by the reduced scattering coefficient, given as μ′s = μs(1 – g1), where g1 =< cos(θ) > is the scattering anisotropy; light transport at these length scales in tissue is considered diffuse and is insensitive to the exact shape of the PF. However, reflectance intensities collected by optical devices with small source-detector separations contain contributions from non-diffuse photons, making the collected intensity dependent on both μs and the exact form of the PF [4, 5, 6, 7]. Failure to account for PF effects for small source-detector separations will introduce errors into optical property estimates. Previous investigations of light transport near the source [8, 9, 10, 11] utilized the Legendre moments of the PF to characterize the effect of large angle scattering events on the collected reflectance signal, introducing a parameter
| (1) |
that includes information about the first two moments of the PF, given as g1 and g2, respectively. These first two moments correspond to the mean and the variance of the distribution of the angular scattering events specified by the PF. These mathematical approaches have been shown to be valid for source-detector separations that are greater than 0.5 mm [9].
Our group has focused on developing quantitative fiber optic devices that utilize a single optical fiber to deliver and collect light during measurement of a turbid medium, such as tissue. Light transport relevant to single fiber reflectance (SFR) measurements, where source and detector spots overlap, is not described by existing analytical models. Recently, our group introduced a semi-empirical model that describes the PF-dependent relationship between the collected SFR intensity and the dimensionless reduced scattering, a term given as the product of the reduced scattering coefficient and the fiber diameter (μ′sdfiber) [12]; from this relationship it is important to note that variations in μ′s and dfiber interchangeably affect the collected light intensity. In a subsequent study, our semi-empirical model was further refined to characterize the PF-dependence of the SFR intensity in terms of μ′sdfiber and γ [13]. The application of this model to analyze SFR spectra measured in tissue is complicated because the tissue PF, and in turn γ, is not well characterized, and have been reported to be wavelength-dependent [10]. Our previous study [13] showed the potential of utilizing multiple SFR spectra measured by different fiber diameter probes to characterize the tissue scattering properties without any a priori information about the tissue PF. Specifically, a multiple diameter single fiber reflectance (MDSFR) approach utilizes the SFR intensity vs. fiber diameter profiles to inform independent estimation of the effects of μ′s and γ on the reflectance signal.
In the present study we characterize the sensitivity of MDSFR estimates of μ′s and γ on the fiber diameters included in the MDSFR approach and on constraints within the analysis algorithm. To investigate this, a Monte Carlo model was utilized to simulate SFR measurements over a wide range of scattering coefficients relevant for tissue (μ′s ∈ [0.4 – 4] mm−1) [14, 15] and biologically relevant phase functions (Modified Henyey-Greenstein phase function with g1 ∈ [0.8 – 0.95] and γ ∈ [1.4 – 1.9]) [8], for 7 fiber diameters (dfiber ∈ [0.2,0.3,0.4,0.6,0.8,1.0,1.5] mm). Simulated measurements are used to characterize the influence that the number of fibers and the range of fiber diameters included in the MDSFR analysis have on the accuracy of the estimated scattering parameters. Also, this study considers the potential benefit of a priori specification of wavelength-dependent scattering constraints on MDSFR accuracy.
2. Materials and methods
2.1. Single fiber reflectance model
Figure 1 shows a schematic of the SFR spectroscopy setup. During measurement, photons are emitted from a white light source and guided through the single fiber probe to the fiber tip where they enter the medium under investigation. Within the medium photons are scattered and absorbed and a fraction of the photons is reflected back into the single fiber and collected by the spectrometer.
Fig. 1.
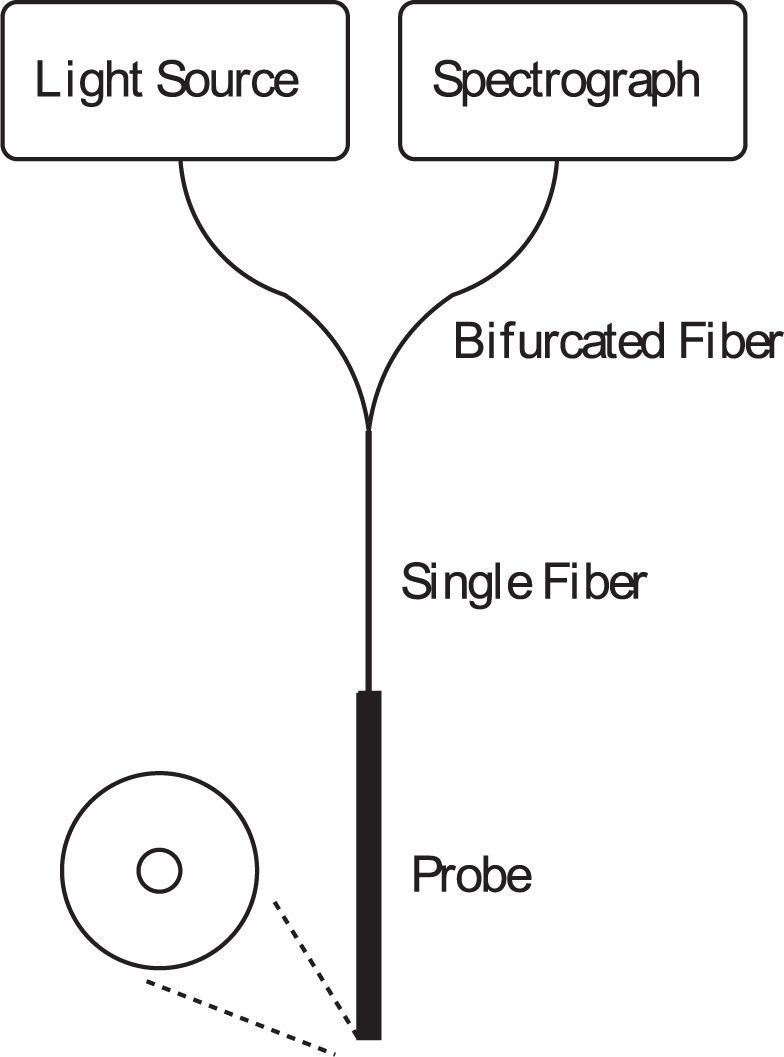
Single fiber reflectance spectroscopy setup.
Quantitative analysis of a measured single fiber reflectance spectrum (RSF) requires the use of an empirical model that describes the wavelength-dependent collected light intensity in the absence of absorption ( ), and applies a Modified Beer-Lambert law to that profile to account for absorption from tissue chromophores:
| (2) |
Here, is the SFR intensity in the absence of absorption, and <LSF> is the effective SFR photon path length, which has a dependence on the sampled optical properties that has been fully characterized previously as [16, 17]:
| (3) |
where CPF is a coefficient that describes the effect of phase function on 〈LSF〉, described previously [16]. Recently, our group introduced a semi-empirical model [12, 13] that describes the relationship between and the dimensionless reduced scattering μ′sdfiber and γ, given as:
| (4) |
Here, the product of the terms preceding the square brackets represents the single fiber collection efficiency, where ηlimit is the diffuse limit, which approximates to be 2.7% for a single fiber with NA = 0.22 [12, 18]. The term within the brackets describes the saturating relationship between and μ′sdfiber that showed phase function independent behavior for high dimensionless scattering values (μ′sdfiber ≥ 10) [12]. This expression accurately describes over a wide range of μ′s ∈ [0.4 – 4] mm−1, anisotropy g1 ∈ [0.8 – 0.95], and γ ∈ [1.4 – 1.9] for the Modified Henyey-Greenstein phase function.
2.2. Monte Carlo simulations of single fiber reflectance
The Monte Carlo code utilized in this study is based on the MCML approach to stochastically simulate photon propagation within a turbid medium [19]. The code was adapted to mimic a single fiber measurement of a turbid medium with homogeneously distributed optical properties; this model has previously been described in detail [12, 16] and will here only be introduced briefly. Photons were initialized by selecting a location on the fiber face in contact with the medium and were launched into a direction within the fiber cone of acceptance; both location and direction were sampled from uniform distributions. The index of refraction n of the medium was set to 1.37 [20, 21] and that of the fiber to 1.5. Reflection and refraction at the boundary between fiber face and medium were accounted for by using Fresnel’s equation and Snell’s law. The photons propagated through the medium in discrete steps selected from an exponential distribution that was weighted by the scattering coefficient. Each scattering event resulted in a change in propagation direction that was sampled from the user-specified scattering phase function. Simulations returned collected reflectance intensity (in the absence of absorption) in units of percentage of incident photons.
In this study the Monte Carlo model simulations were performed to mimic measurements of SFR spectra on turbid media that contain biologically-relevant scattering properties. While individual simulations return the equivalent of a measured intensity at a single wavelength, the range of optical properties within the set of simulations was selected such that combinations of individual simulations could be used to represent reflectance spectra measured in a turbid medium. For each group of simulations that represent a measured spectrum, the wavelength dependence of μ′s (λ) was assumed to follow Mie scattering, as
| (5) |
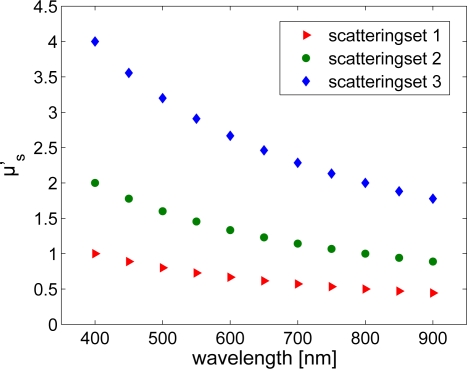
where a1 and a2 represent the Mie scattering amplitude and scattering power, respectively, and λo represents a normalization wavelength (chosen to be 800 nm in this study). Here, μ′s(λ) was calculated for λ ∈ [400 – 900] nm in 50 nm increments with a2 = −1.0 (which is typical for tissue [22]) and a1 adjusted to calculate three different sets of scattering profiles, such that μ′s (800nm) = [0.5,1.0,2.0] mm−1 respectively; this yielded 33 μ′s values in total. Figure 2 shows the 3 individual scattering sets utilized in this study.
Fig. 2.
Wavelength dependent reduced scattering coefficient for the 3 scattering sets considered in simulations.
Each μ′s value investigated in this study was constructed independently for 3 different anisotropies (g1 ∈ [0.8,0.9,0.95]) that were derived from the Modified Henyey-Greenstein (MHG) phase function:
| (6) |
The MHG phase function is a combination of the Henyey-Greenstein phase function (pHG) and an isotropic component that contains the cos2(θ) term. By choosing specific values for gHG and the normalization parameter α the phase functions were constructed in a way that its second Legendre moment, g2, led to values of γ that encompassed the biologically relevant range (γ ∈ [1.4,1.5,1.6,1.7,1.8,1.9] as reported in previous literature [6]. In total 17 phase functions were implemented for all possible combinations of g1 and γ with the exception of g = 0.8 and γ = 1.9; such a combination cannot be obtained with Eq. (6). SFR measurements of the 561 combinations of optical properties were each simulated for 7 different fiber diameters (dfiber ∈ [0.2,0.3,0.4,0.6,0.8,1.0,1.5] mm) leading to a total amount of 3927 simulated SFR signals. Each simulation launched at least 2 × 106 photons.
2.3. Estimation of μ′s and γ from MDSFR measurements
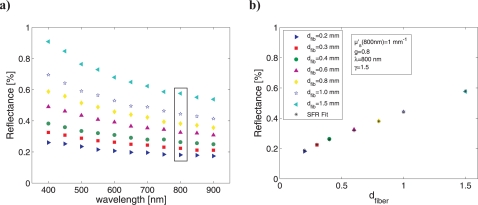
As reported previously [13], the MDSFR approach is based on the principle that SFR measurements of a turbid medium by multiple different fiber diameter probes will yield a PF-specific vs. dfiber relationship (as visualized in Fig. 3a) that allows the estimation of both μ′s(λ) and γ(λ). This theory applies to measurements at a single wavelength, assuming that the measurements are in the absence of absorption such that RSF approximates to ; the application of Eq. (4) to the data will yield estimates of μ′s(λ) and γ(λ) at one selected wavelength if at least 3 fiber diameters are measured. However, analysis of measurements made in the presence of absorption necessitate the estimation of over the spectral wavelength range prior to application of Eq. (4), a calculation that requires specification of a wavelength-dependent function for μ′s(λ) as well as specification of a set of chromophores and their molecular extinction coefficients contributing to μa(λ), as will be discussed extensively in Section 4. One candidate background scattering model in tissue is the Mie scattering relationship given in Eq. (5) [23, 24]. Instead of fitting the values for μ′s and γ for each wavelength separately, the introduction of a background scattering model as a spectral constraint allows to fit data measured at multiple wavelengths and multiple fiber diameters simultaneously. In this case, the model Eq. (4) can be rewritten in a matrix representation, as:
| (7) |
where i and j represent indices for sampled λ and dfiber, and a1, a2 and γi are free fit parameters. For example, for a simulated MDSFR tissue measurement comprising 11 wavelengths λi and 7 fiber diameters , the data set consists of 77 values and the model to which these data are fit (Eq. (7)) contains 13 free fit parameters (a1, a2 and 11 γi values). The MDSFR analysis algorithm estimates μ′s(λ) (or a1 and a2 in case of a spectrally constrained fit) and γ(λ) by minimizing the weighted residual error between the simulated and the model estimated for all included fiber diameters (and all included wavelengths in case of a spectrally constrained fit) simultaneously. The fitting routine utilizes a Levenberg-Marquardt algorithm coded into a Matlab script (version R2009, MathWorks). Confidence intervals of the estimated parameters were calculated from the square root of the diagonal of the covariance matrix, as described previously [25]. Note that an additional practical benefit gained from the spectrally resolved MDSFR analysis, is that the fit only requires 2 fiber diameters, as opposed to a minimum of 3 diameters if μ′s(λ) and γ(λ) are fitted at a single wavelength.
Fig. 3.
a) Simulated MDSFR reflectance measurements for 7 fiber diameters as a function of wavelength, and b) simulated and fitted reflectance at a single wavelength (800 nm) as a function of fiber diameter.
2.4. MDSFR sensitivity analysis to fiber diameter set
Simulated MDSFR measurements for each of the 561 combinations of scattering properties were utilized to determine the accuracy of μ′s(λ) and γ(λ) estimated at a single wavelength. In order to guide the design of the number and size of fibers to be included in MDSFR approach, the accuracy of parameter estimates were evaluated by systematically removing reflectance intensities corresponding to individual fibers from each MDSFR measurement at a single wavelength and calculating the resulting introduction of error. The accuracy of the fit was analyzed A) when the range of fiber diameters is decreased by subsequently removing data from larger fibers, B) when the range of fiber diameters is decreased by subsequently removing data from smaller fibers, and C) when intermediate fiber diameters are removed, while maintaining data from the largest and the smallest fiber diameters. In all 3 of the above cases, stochastic noise of 5% was introduced to the measured reflectance intensity data and the fit procedure was repeated 100 times; mean residual errors between model estimates and simulated values of μ′s and γ (calculated as and , respectively) as well as standard deviations calculated from repeated sets of error estimates are reported throughout this paper.
To investigate the application of the MDSFR approach to spectral measurements in tissue, a spectrally resolved MDSFR analysis was conducted in the presence of wavelength-dependent changes in γ(λ). The relationship between γ and wavelength in tissue has not yet been elucidated and therefore cannot be informed by any set of specified spectral constraints; while wavelength-dependent changes are expected [10], the exact form of these changes is unknown. To consider how different wavelength-dependent changes in γ may influence the MDSFR performance, 3 different scenarios were analyzed: I) assuming γ(λ) constant over the wavelength range, II) assuming γ(λ) monotonically decreasing with wavelength, and III) assuming γ(λ) random for each wavelength. In case I analysis was performed for each of the 17 PF-specific scattering constructions of each of the 3 scattering sets, in case II γ was varied from 1.9 to 1.4, and g1 values varied in the set ∈ [0.95,0.9,0.8], across the 400 – 900 nm wavelength range (with a 0.1 step decrease in γ per 100 nm, and a decrease in g1 introduced every 200 nm), and in case III 10 data sets were constructed with randomly selected γ and g1 values at each wavelength. These test scenarios were performed using information from all 7 fiber diameters. The influence of reducing fiber diameters on the spectrally resolved calculation (with a specific focus on the error associated with probes containing 2 fiber diameters) was performed with γ(λ) constant over the wavelength range.
3. Results
3.1. Accuracy of single wavelength MDSFR analysis
Figure 3a shows the SFR intensity vs. wavelength data for a MDSFR measurement with 7 fiber diameters (range 0.2 – 1.5 mm) on a turbid medium with μ′s = 1 mm−1 at 800 nm (with the wavelength dependence of μ′s described by scattering set 2), with g1 = 0.8 and γ = 1.5 at all wavelengths. Here, symbols differentiate between measurements made by different fiber diameters. These data represent SFR intensity measured in the absence of absorption ( ). The MDSFR method utilizes the vs. dfiber relationship measured at each individual wavelength to extract estimates for both μ′s(λ) and γ(λ). Figure 3b shows the vs. (dfiber relationship at 800 nm; simulated data are fit to Eq. (4). MDSFR analysis yields estimates of reflectance intensity in good agreement with simulated data (visualized on the graph), and also yields estimates of μ′s(800 nm) and γ(800 nm), which are respectively given as 0.99 mm−1 (true value of 1.0 mm−1) and 1.46 (true value of 1.5).
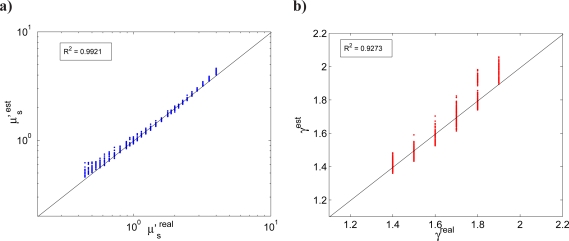
The MDSFR single wavelength analysis was applied to all simulated combinations of scattering properties. Figure 4 shows the comparison of estimated vs simulated values for μ′s and γ; these calculations utilized all 7 simulated fiber diameters in the MDSFR fitting procedure. Here, both scattering parameters were accurately estimated over a wide range of reduced scattering coefficients (0.4 < μs 0 < 4 mm−1) and for all 17 phase functions and for all 11 wavelengths (λ = [400 – 900] nm) with an average mean residual error for μ′s of 4.9% and for γ of 2.5%.
Fig. 4.
a) MDSFR estimated vs. simulated μ′s, and b) MDSFR estimated vs. simulated γ for the entire data set for all 11 wavelengths (λ = 400 – 900 nm). Calculations utilized 7 fiber diameters in the fitting procedure in the range dfiber ∈ [0.2 – 1.5] mm.
3.2. Sensitivity of single wavelength MDSFR analysis to fiber diameter set
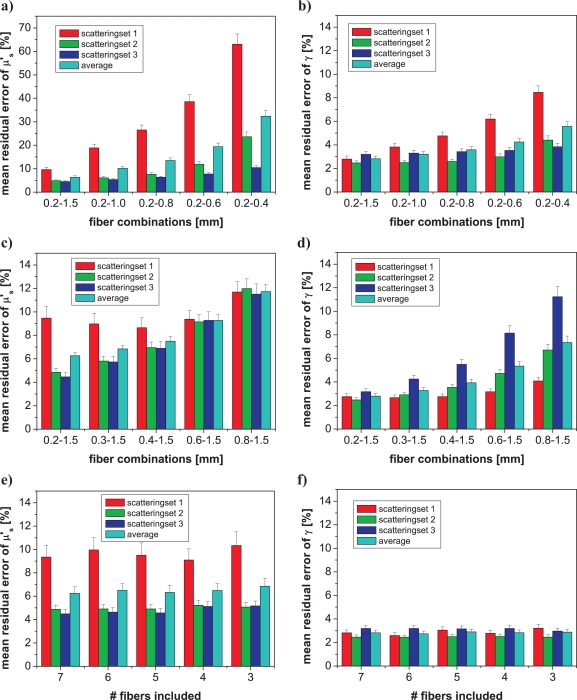
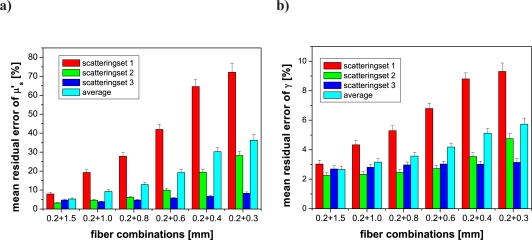
This study characterized the sensitivity of scattering parameter estimates to the fiber diameters included in the MDSFR analysis. In Fig. 5 the abscissa of the graphs denotes the combinations of fiber diameters that contribute to the fitted MDSFR analysis and the ordinate denotes the mean residual error of either μ′s or γ. The colors of the bars denote the scattering ranges with red corresponding to scattering set 1 (range μ′s =[0.4 – 1] mm−1), green corresponding to scattering set 2 (range μ′s =[0.9 – 2] mm−1), blue corresponding to scattering set 3 (range μ′s =[1.8 – 4] mm−1) and cyan corresponding to the entire data set (Fig. 2). The error bars display the standard deviation of the 100 repetitioned fits with a random noise of 5% introduced to the data. Figs. 5a and b show the influence of removing large fiber diameters from the 7 fibers included in the full MDSFR data set on the mean residual error in μ′s and γ, respectively. Conversely, Figs. 5c and d display the effect of removing small fibers. The first set of columns in panels a–f show the case when all fibers are included into the fitting routine. It is observed that the mean residual error of μ′s is highest for scattering set 1 (lowest scattering); the error in μ′s estimation increases substantially for these low scattering coefficients when large fibers are subsequently removed from the dataset, as observed in Fig. 5a. The effect of fiber removal on estimation of γ follows a similar trend (see Fig. 5b). Alternatively, when small fibers are removed from the basis set of fibers included in the MDSFR fitting routine, the resulting mean residual error for μ′s approaches 12% for all scattering sets, as shown in Fig. 5c; this error is substantially less than the case when larger fibers were removed, which depends on the magnitude of μ′s and approached 60% for the low scattering set (set 1). For the removal of small fibers, the mean residual error in γ increased for all data from 3% to 7%, and also showed an increased error associated with high scattering samples, given as scattering set 3; this is visualized in Fig. 5d. Such a result is consistent with the understanding that measurements of SFR intensity in samples with high μ′sdfiber become independent of γ [12].
Fig. 5.
Effect of different fiber diameter combinations on the mean residual error of μ′s and γ estimated by single wavelength MDSFR analysis. a) and b) shows subsequent removal of large fibers; all remaining smaller fibers were included. c) and d) shows subsequent removal of small fibers; all remaining larger fibers were included. e) and f) shows removal of intermediate fibers; reflectance data from 0.2 mm and 1.5 mm fibers were always included. Note the difference in y-axis scale between panels a and b–f. Scattering set 1: μ′s = 0.4 – 1.0 mm−1; scattering set 2: μ′s = 0.9 – 2.0 mm−1; scattering set 3: μ′s = 1.8 – 4.0 mm−1.
Panels e and f of Fig. 5 describe the influence of removing intermediate fiber diameters from the MDSFR fiber set. In these cases each analysis retained fiber diameters of 0.2 and 1.5 mm, while differing quantities of the intermediate fibers were randomly removed. Inspection of panels e–f show a minimal influence of the removal of the intermediate fibers on MDSFR performance, with very minor changes to the mean error observed for either the entire data set or each individual scattering set. This behaviour was maintained when the simulated measurement noise level was increased from 5% to 10% or 20%; in this case the mean residual error increased similarly for all fiber combinations and scattering sets (data not shown). These results indicate that the accuracy of μ′s and γ estimates are influenced by the range of diameters, and in turn the range of μ′sdfiber, included in the MDSFR analysis, and not the quantity of the intermediate fibers.
3.3. Accuracy of spectrally-resolved MDSFR analysis
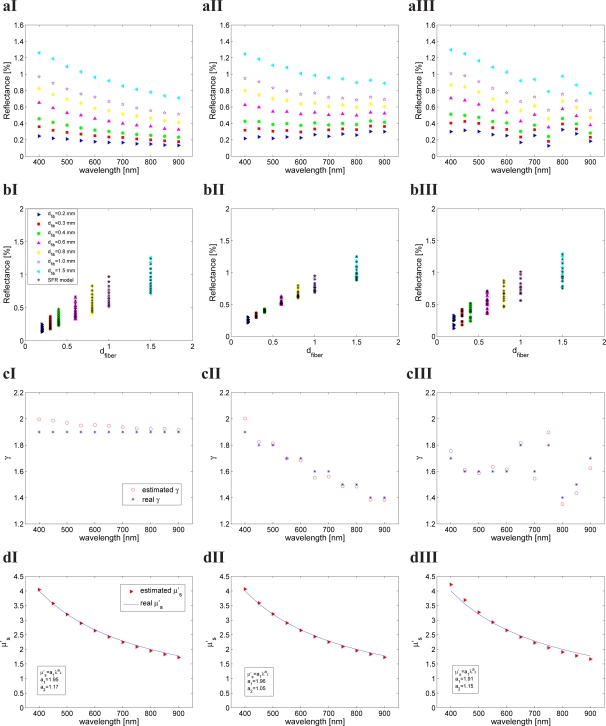
Figures 6a-I, -II and -III show vs. wavelength data for MDSFR measurements with 7 fiber diameters (range 0.2 – 1.5 mm) on a turbid medium with μ′s = 2 mm−1 at 800 nm (with the wavelength dependence of μ′s described by scattering set 3). In Fig. 6a-I, γ and g1 are constant across all wavelengths, with g1 = 0.9 and γ = 1.9. In Fig. 6a-II, the specified PF varies across the 400 – 900 nm wavelength range, with γ varied from 1.9 to 1.4 and g1 values varied in the set ∈ [0.95,0.9,0.8]. In Fig. 6a-III, g1 and γ were randomly selected for each wavelength. Figures 6b-I, -II and -III show vs. fiber diameter for these sets of MDSFR measurements. Application of the spectrally resolved MDSFR fitting method to these measurements yields estimates of reflectance intensity in good agreement with simulated data (visualized on the graph), leading to estimates of γ at each analyzed wavelength, and estimates of scattering model parameters a1 and a2 from Eq. (7). The simulated and fitted wavelength profiles of γ(λ) and μ′s(λ) are displayed in Figs. 6c and 6d, respectively.
Fig. 6.
Example for spectrally resolved MDSFR fitting procedure using data from scattering set 3 (μ′s(800 nm) = 2 mm−1): a) simulated MDSFR intensities for 7 fiber diameters, b) MDSFR intensity vs. fiber diameter for all wavelengths, c) estimated and simulated γ as a function of wavelength and d) estimated and simulated μ′s as a function of wavelength for I) wavelength independent γ and g1, II) wavelength dependent γ and g1 and III) random γ and g1.
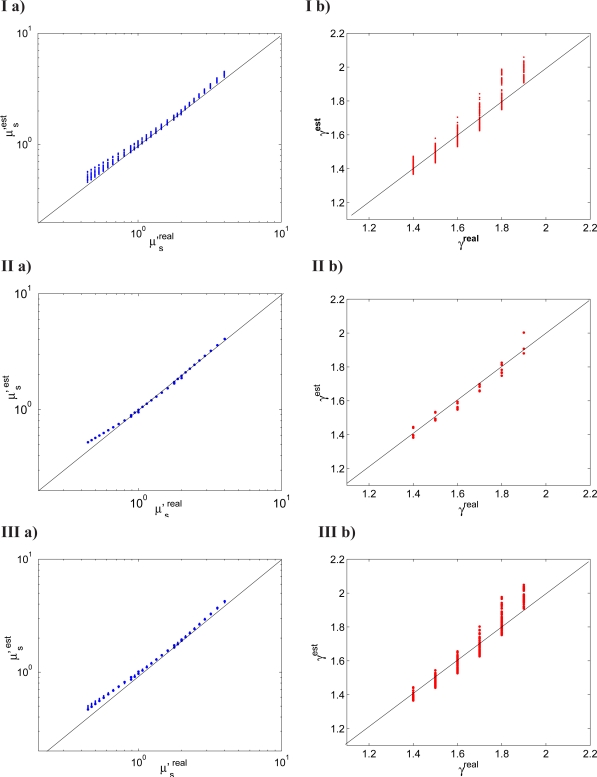
To investigate the potential utility of the MDSFR approach to analyze spectral measurements in tissue, it is important to determine the influence that an unknown and potentially wavelength-dependent PF (and γ) may have on the estimated scattering properties. The spectrally resolved MDSFR approach was evaluated on sets of MDSFR measurements with I) γ and g1 fixed, II) γ(λ) and g1(λ) decreasing monotonically with wavelength, and III) γ(λ) and g1(λ) completely randomized; details of these sets are described in Section 2.4. The results for these cases are shown in Fig. 7. For all three cases both μ′s(λ) and γ(λ) were accurately estimated from the whole dataset, with mean residual errors of ≤ 5% for μ′s and of ≤ 3% for γ.
Fig. 7.
Spectrally resolved MDSFR fitting procedure: a) estimated vs. real μ′s; b) estimated vs. real γ for I) wavelength independent γ and g, II) wavelength dependent γ and g1 and III) random γ and g1. The analysis was performed over the whole dataset including all 3 scattering ranges and all 11 wavelengths (λ = 400 – 900 nm).
3.4. Sensitivity of spectrally-resolved MDSFR analysis to fiber diameter set
The sensitivity of the spectrally resolved MDSFR approach to the variation in the quantity and combination of fiber diameters was investigated. The error profiles resulting from the analysis, described in Section 2.4, were similar to the error profiles presented for the single-wavelength MDSFR analysis in Fig. 5 (data not shown). The novel aspect of investigating the sensitivity of the spectrally resolved MDSFR approach to fiber diameter set is the case involving 2 fiber diameters. In this analysis, pairs of fibers were considered with the the smallest fiber diameter specified for all pairs as 0.2 mm and the larger fiber selected from different diameters. The mean residual errors for this analysis are shown in Fig. 8. Here, combinations of small fiber diameters are associated with increased error; this trend is substantially increased for measurements in the low scattering regime (set 1). Interestingly, measurements of the high scattering case (set 3) yielded accurate estimates even for measurements with the 0.2 and 0.3 mm fiber combination. These results highlight the finding that error in the estimated scattering properties is dependent on the range of dimensionless reduced scattering values included within the MDSFR analysis.
Fig. 8.
Sensitivity of spectrally resolved MDSFR estimates of a) μ′s and b) γ to the choice of fiber diameters within a 2 fiber MDSFR probe. Data from all 11 wavelengths (λ = 400 – 900 nm) were included.
4. Discussion
This study investigated the sensitivity of μ′s(λ) and γ(λ) estimated by MDSFR to the choice of fibers included in the analysis and to the incorporation of spectral information into the fitting algorithm. Results indicate that μ′s(λ) and γ(λ) estimates are sensitive to the range of fiber diameters but not the quantity of intermediate fibers. Results also show that the MDSFR approach does not require any a priori knowledge of the PF within the sampled medium.
4.1. Influence of fiber diameters included in MDSFR analysis
This study investigated the influence of the number and diameter of fibers included in the MDSFR analysis on the accuracy of estimated scattering parameters. One unique aspect of the MDSFR analysis is that SFR intensity depends on μ′sdfiber, an important factor that indicates that the theory can be applied interchangeably to different sets and combinations of fiber diameters. Results indicated that the accuracy of μ′s and γ estimates depends on the range of fiber diameters included in the analysis; specifically the range of μ′sdfiber is important. This finding is supported by the high degree of accuracy in μ′s and γ estimates obtained from MDSFR analysis that included 7 fibers over the range of diameters [0.2 – 1.5] mm for all 3 scattering sets investigated (as shown in Fig. 5). It is observed that reduction in the range of fibers introduced error, with substantial increases in error associated with measurement of small scattering coefficients by combinations of small diameter fibers (as evident in Fig. 5a–b). Interestingly, the presence of intermediate fibers did not alter error in parameter estimates. These observations lead to the following heuristic guidelines: for tissues with medium to high scattering coefficients (μ′s > 1 mm−1), an MDSFR measurement with a relatively limited number of small fiber diameters suffices to accurately extract μ′s and γ, while for tissues with low scattering coefficients (μ′s < 1 mm−1) larger fiber diameter measurements need to be included in the data set to accurately extract μ′s and (to a lesser extent) γ. These heuristic guidelines can be used to evaluate the performance of potential MDSFR devices designed for specific clinical application areas, e.g. MDSFR devices compatible with endoscopic applications, where the largest allowable single fiber included in the MDSFR measurement is dfiber < 2 mm, or MDSFR devices compatible with Fine Needle Aspiration (FNA) procedures, where the MDSFR technique is performed through the lumen of an FNA needle and the largest single fiber included in the MDSFR measurement is dfiber < 0.45 mm. The sensitivity results indicate that for endoscopic applications, μ′s and γ can be extracted with an accuracy of better than 10% by using 0.2 mm and 1.5 mm fibers while employing a spectrally resolved MDSFR fitting procedure; it is possible to perform a wavelength independent assessment of μ′s and γ if intermediate fiber diameters are included in the MDSFR measurement. For FNA applications, μ′s and γ can be extracted with an accuracy of better than 20% and 10%, respectively, as long as μ′s > 1 mm−1. If μ′s < 1 mm−1, the accuracy in μ′s estimation reduces dramatically, while retaining the high accuracy of γ estimation. The clinical importance of the reported errors depends on the biology of the intended clinical application, e.g. on the difference between the scattering properties of healthy and (pre)malignant tissues.
4.2. Assessment of model error
Inspection of MDSFR estimates of μ′s and γ show increased error in the low dimensionless scattering regime, an increase that is attributable to model error. Inspection of the formulation of Eq. (4) shows two potential sources or error: first, the fitted coefficients reported in Eq. (4) were informed by a wide range of reduced scattering coefficients ([0.1 – 200] mm−1), such that SFR data in the low μ′sdfiber regime, where PF has the greatest influence on the SFR intensity, represented a subset of the total fitted parameter space [12]. Second, the characterization of the PF influence on SFR intensity was expressed concisely in terms of a single parameter γ [13]. While the resulting formulation of Eq. (4) yields high quality estimates of μ′s over a wide range of values, it is possible that higher order moments of the PF exclusively contribute to SFR intensity at low μ′sdfiber. These model errors can be minimized by simulating measurements that resample the SFR intensity at low μ′sdfiber values and re-evaluate the fitted coefficients in Eq. (4). However, such an analysis is beyond the scope of the current paper and will be the focus of future work.
4.3. Comparison of MDSFR analysis at a single wavelength or multiple wavelengths: influence of absorption
This study utilized two approaches to analyze MDSFR measurements: (1) a single wavelength MDSFR analysis, which extracts μ′s and γ independently for each wavelength; (2) a spectrally-resolved MDSFR analysis that utilizes prior knowledge of the wavelength dependence of μ′s as a constraint to fit μ′s and γ over a selected wavelength range. Comparison of sensitivity results from each method showed similar levels of accuracy in estimated parameter values; however, proper application of these methods to measurements of tissue requires careful consideration of the underlying assumptions.
The single-wavelength MDSFR analysis requires the measured reflectance intensity to be made in the absence of absorption; this is important because the influence of absorption on the SFR intensity will affect the reflectance intensities, measured with each fiber diameter differently, as SFR photon path length depends on the fiber diameter [16]. Note that the influence of absorption on SFR intensity cannot be resolved at a single wavelength. For measurements in tissue, the single wavelength MDSFR analysis may still be valid if performed at a wavelength where background absorption due endogenous chromophores (e.g. blood) is minimal. Considering MDSFR measurements in tissue, containing vascular parameters of 2% blood volume fraction and 70% microvascular saturation, analysis at a wavelength of 800 nm would introduce only a 0.5% and 2.6% attenuation of the measured reflectance of the 0.2 mm and 1.5 mm fibers, respectively. This would result in only small increases of the error in estimation of μ′s and γ (from 6.0% to 6.2% and 2.57% to 2.63%, respectively). However, the associated error will depend on the actual blood content, oxygenation, wavelength as well as scattering coefficient; factors that cannot be resolved at a single wavelength.
The wavelength constrained MDSFR method can extract μ′s and γ values over the whole wavelength range even in presence of strong absorption. As we have described previously [16, 12, 17], the SFR spectrum in the presence of absorption RSF in its most general form can be described by combining Eqs. (2)–(4):
| (8) |
| (9) |
Note that the general form of Eq. (4), which is valid for a wider range of phase functions than the MHG phase function utilized in this paper, contains phase function dependent parameters [ρ1,ρ2,ρ3] and does not express these parameters in terms of γ [12]. Furthermore, the path length Eq. (9) contains a phase function dependent constant CPF. We have previously reported that substituting the parameter set [ρ1,ρ2,ρ3,CPF] by the values [6.82, 0.97, 1.55, 0.944] enables accurate extraction of from SFR spectra measured in turbid media without a priori knowledge of either μ′s or phase function [17]. The tissue absorption coefficient is the sum of the absorption coefficients of all the chromophores present in the interrogation volume, which can be written as:
| (10) |
Here ck and ɛk are the concentration and extinction coefficient, respectively, of absorbing molecules (index k) such as oxyhemoglobin, deoxyhemoglobin, bilirubin, beta-carotene, lipids and water. Note that Eq. (10) may be modified to contain correction factors for the inhomogeneous distribution of absorbing molecules such as blood [26]. Application of Eqs. 8 and 9 to SFR spectra measured by each independent fiber diameter returns estimates of ck specific for each sampled fiber. While this calculation requires the specification of a background scattering model, it is important to note that the fit returns independent of fitted scattering parameters. The resulting absorption coefficient estimates can be used to estimate reflectance spectra in the absence of absorption , as
| (11) |
Finally, the MDSFR fitting routine as described in Section` 2.3 can be applied to to obtain estimates for μ′s and γ over the whole wavelength range.
For the case where absorbers are distributed heterogeneously (e.g., layered tissue), the above calculation will allow fiber diameter specific estimation of , that may be necessary due to slightly different sampled volumes between fiber diameters [16]; it is important to note that the MDSFR calculation assumes that scattering properties do not substantially differ between the sampled volume(s); the validity of this assumption will be investigated in future work.
We would like to emphasize that broadband MDSFR measurements of reflectance spectra of tissue in the ultraviolet-visible (UV-VIS) wavelength range will require spectrally resolved analysis that introduces spectral constraints regarding the form of wavelength-dependent changes in μ′s. Previous investigations that characterized μ′s vs. wavelength in the UV-VIS range in tissue identified the presence of a combination of Mie and Rayleigh scattering that is sufficiently described by a power-law function. However, the specification of the background model structure is a potential limitation to the general use of the MDSFR technique, as the extracted scattering information is not allowed to deviate from the specified functionality; this constraint may prevent identification of subtle wavelength-dependent influences of scattering on the reflectance signal. The appropriateness of the background scattering model becomes increasingly important for MDSFR measurements made with very small fibers, including diameters smaller than those investigated in the current study, where the collected signal will contain an increasingly dominant non-diffuse component; these issues will be addressed in future studies.
5. Conclusion
MDSFR provides a method to accurately determine both μ′s(λ) and the parameter characterized by the first two moments of the PF, γ(λ), within a turbid medium; the approach was shown to be valid over a broad range of optical properties that are representative of biological tissue. Results presented in this study identified the sensitivity of MDSFR estimates to the range of optically sampled μ′sdfiber, and the insensitivity of model estimates to the quantity of intermediate fibers included in the analysis. Additionally, the MDSFR does not require any a priori knowledge of the wavelength-dependent changes in γ. These results indicate that the MDSFR approach can return a local quantitative assessment of μ′s that is independent of the tissue PF. This is a substantive improvement over many previous fiber optic approaches to estimating μ′s at small source detector separations that did not elucidate the PF-influence on the collected reflectance [27, 28, 29, 30, 31, 32], and therefore, are likely to be less accurate in environments with unknown (or wavelength dependent) PF [4, 9, 7]. The MDSFR approach extracts information about both μ′s and the PF from reflectance intensities measured over multiple length scales, in the form of multiple fiber diameters; the basis of this approach is similar to the spatially-resolved method described previously by Bevilacqua et al [6, 8]. Application of this approach to in vivo tissue may yield PF-information that provides novel insight into tissue morphology and ultrastructure; information that may have potential diagnostic utility [10].
This study utilized simulated data to identify heuristic rules to guide the design of MDSFR fiber probes for specific clinical applications. Studies are ongoing that will validate the approach by experimental measurement in optical phantoms. Further work will investigate the effect of inhomogeneous scattering distributions on MDSFR performance, and utilize the technique to characterize scattering properties in tissue in vivo.
References and links
- 1.Boustany N., Boppart S., Backman V., “Microscopic Imaging and Spectroscopy with Scattered Light,” Annu. Rev. Biomed. Eng. 12, 285–314 (2010). 10.1146/annurev-bioeng-061008-124811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drezek R., Guillaud M., Collier T., Boiko I., Malpica A., Macaulay C., Follen M., Richards-Kortum R., “Light scattering from cervical cells throughout neoplastic progression: influence of nuclear morphology, DNA content, and chromatin texture,” J. Biomed. Opt. 8, 7–16 (2003). 10.1117/1.1528950 [DOI] [PubMed] [Google Scholar]
- 3.Georgakoudi I., Van Dam J., “Characterization of dysplastic tissue morphology and biochemistry in Barrett’s esophagus using diffuse reflectance and light scattering spectroscopy,” Tech. Gastrointest. Endosc. 7, 100–105 (2005). 10.1016/j.tgie.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 4.Mourant J., Boyer J., Hielscher A., Bigio I., “Influence of the scattering phase function on light transport measurements in turbid media performed with small source-detector separations,” Opt. Lett. 21, 546–548 (1996). 10.1364/OL.21.000546 [DOI] [PubMed] [Google Scholar]
- 5.Canpolat M., Mourant J., “High-angle scattering events strongly affect light collection in clinically relevant measurement geometries for light transport through tissue,” Phys. Med. Biol. 45, 1127–1140 (2000). 10.1088/0031-9155/45/5/304 [DOI] [PubMed] [Google Scholar]
- 6.Bevilacqua F., Depeursinge C., “Monte Carlo study of diffuse reflectance at source–detector separations close to one transport mean free path,” J. Opt. Soc. Am. A 16, 2935–2945 (1999). 10.1364/JOSAA.16.002935 [DOI] [Google Scholar]
- 7.Kienle A., Forster F., Hibst R., “Influence of the phase function on determination of the optical properties of biological tissue by spatially resolved reflectance,” Opt. Lett. 26, 1571–1573 (2001). 10.1364/OL.26.001571 [DOI] [PubMed] [Google Scholar]
- 8.Bevilacqua F., Piguet D., Marquet P., Gross J., Tromberg B., Depeursinge C., “In vivo local determination of tissue optical properties: applications to human brain,” Appl. Opt. 38, 4939–4950 (1999). 10.1364/AO.38.004939 [DOI] [PubMed] [Google Scholar]
- 9.Hull E., Foster T., “Steady-state reflectance spectroscopy in the P3 approximation,” J. Opt. Soc. Am. A 18, 584–599 (2001). 10.1364/JOSAA.18.000584 [DOI] [Google Scholar]
- 10.Thueler P., Charvet I., Bevilacqua F., Ghislain M., Ory G., Marquet P., Meda P., Vermeulen B., Depeursinge C., “In vivo endoscopic tissue diagnostics based on spectroscopic absorption, scattering, and phase function properties,” J. Biomed. Opt. 8, 495–503 (2003). 10.1117/1.1578494 [DOI] [PubMed] [Google Scholar]
- 11.Tian H., Liu Y., Wang L., “Influence of the third-order parameter on diffuse reflectance at small source-detector separations,” Opt. Lett. 31, 933–935 (2006). 10.1364/OL.31.000933 [DOI] [PubMed] [Google Scholar]
- 12.Kanick S. C., Gamm U. A., Schouten M., Sterenborg H. J. C. M., Robinson D. J., Amelink A., “Measurement of the reduced scattering coefficient of turbid media using single fiber reflectance spectroscopy: fiber diameter and phase function dependence,” Biomed. Opt. Express 2, 1687–1702 (2011). 10.1364/BOE.2.001687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanick S. C., Gamm U. A., Sterenborg H. J. C. M., Robinson D. J., Amelink A., “Method to quantitatively estimate wavelength-dependent scattering properties from multi-diameter single fiber reflectance spectra in a turbid medium,” Opt. Lett. 36, 2997–2999 (2011). 10.1364/OL.36.002997 [DOI] [PubMed] [Google Scholar]
- 14.Cheong W.F., Prahl S.A., Welch A.J., “A review of the optical properties of biological tissues,” IEEE J. Quantum Elect. 26, 2166–2185 (1990). 10.1109/3.64354 [DOI] [Google Scholar]
- 15.Salomatina E., Jiang B., Novak J., Yaroslavsky A. N., “Optical properties of normal and cancerous human skin in the visible and near-infrared spectral range,” J. Biomed. Opt. 11, 064026 (2006). 10.1117/1.2398928 [DOI] [PubMed] [Google Scholar]
- 16.Kanick S. C., Robinson D. J., Sterenborg H. J. C. M., Amelink A., “Monte Carlo analysis of single fiber reflectance spectroscopy,” Phys. Med. Biol. 54, 6991–7008 (2009). 10.1088/0031-9155/54/22/016 [DOI] [PubMed] [Google Scholar]
- 17.Kanick S. C., Robinson D. J., Sterenborg H. J. C. M., Amelink A., “Method to quantitate absorption coefficients from single fiber reflectance spectra without knowledge of the scattering properties,” Opt. Lett. 36, 2791–2793 (2011). 10.1364/OL.36.002791 [DOI] [PubMed] [Google Scholar]
- 18.Bargo P., Prahl S., Jacques S., “Collection efficiency of a single optical fiber in turbid media,” Appl. Opt. 42, 3187–3197 (2003). 10.1364/AO.42.003187 [DOI] [PubMed] [Google Scholar]
- 19.Wang L., Jacques S., Zheng L., “MCML–Monte Carlo modeling of light transport in multi-layered tissues,” Comp. Meth. Prog. Biomed. 47, 131–146 (1995). 10.1016/0169-2607(95)01640-F [DOI] [PubMed] [Google Scholar]
- 20.Knüttel A., Boehlau-Godau M., “Spatially confined and temporally resolved refractive index and scattering evaluation in human skin performed with optical coherence tomography,” J. Biomed. Opt. 5, 83–92 (2000). 10.1117/1.429972 [DOI] [PubMed] [Google Scholar]
- 21.Dirckx J. J. J., Kuypers L. C., Decraemer W. F., “Refractive index of tissue measured with confocal microscopy,” J. Biomed. Opt. 10, 044014 (2005). 10.1117/1.1993487 [DOI] [PubMed] [Google Scholar]
- 22.Xu M., Alfano R. R., “Fractal mechanisms of light scattering in biological tissue and cells,” Opt. Lett. 30, 3051–3053 (2005). 10.1364/OL.30.003051 [DOI] [PubMed] [Google Scholar]
- 23.Bevilacqua F., Berger A., Cerussi A., Jakubowski D., Tromberg B., “Broadband absorption spectroscopy in turbid media by combined frequency-domain and steady-state methods,” Appl. Opt. 39, 6498–6507 (2000). 10.1364/AO.39.006498 [DOI] [PubMed] [Google Scholar]
- 24.Mourant J., Freyer J., Hielscher A., Eick A., Shen D., Johnson T., “Mechanisms of light scattering from biological cells relevant to noninvasive optical-tissue diagnostics,” Appl. Opt. 37, 3586–3593 (1998). 10.1364/AO.37.003586 [DOI] [PubMed] [Google Scholar]
- 25.Amelink A., Robinson D. J., Sterenborg H. J. C. M., “Confidence intervals on fit parameters derived from optical reflectance spectroscopy measurements,” J. Biomed. Opt. 13, 054044 (2008). 10.1117/1.2982523 [DOI] [PubMed] [Google Scholar]
- 26.van Veen R. L. P., Verkruysse W., Sterenborg H. J. C. M., “Diffuse-reflectance spectroscopy from 500 to 1060 nm by correction for inhomogeneously distributed absorbers,” Opt. Lett. 27, 246–248 (2002). 10.1364/OL.27.000246 [DOI] [PubMed] [Google Scholar]
- 27.Rajaram N., Nguyen T. H., Tunnell J. W., “Lookup table–based inverse model for determining optical properties of turbid media,” J. Biomed. Opt. 13, 050501 (2008). 10.1117/1.2981797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer G., Ramanujam N., “Monte Carlo-based inverse model for calculating tissue optical properties. Part I: Theory and validation on synthetic phantoms,” Appl. Opt. 45, 1062–1071 (2006). 10.1364/AO.45.001062 [DOI] [PubMed] [Google Scholar]
- 29.Sharma D., Agrawal A., Matchette L. S., Pfefer T. J., “Evaluation of a fiberoptic-based system for measurement of optical properties in highly attenuating turbid media,” Biomed. Eng. Online 5, 49 (2006). 10.1186/1475-925X-5-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reif R., A’Amar O., Bigio I., “Analytical model of light reflectance for extraction of the optical properties in small volumes of turbid media,” Appl. Opt. 46, 7317–7328 (2007). 10.1364/AO.46.007317 [DOI] [PubMed] [Google Scholar]
- 31.Wilson R. H., Chandra M., Scheiman J., Simeone D., McKenna B., Purdy J., Mycek M.-A., “Optical spectroscopy detects histological hallmarks of pancreatic cancer,” Opt. Express 17, 17502–17516 (2009). 10.1364/OE.17.017502 [DOI] [PubMed] [Google Scholar]
- 32.Kim A., Roy M., Dadani F., Wilson B., “A fiberoptic reflectance probe with multiple source-collector separations to increase the dynamic range of derived tissue optical absorption and scattering coefficients,” Opt. Express 18, 5580–5594 (2010). 10.1364/OE.18.005580 [DOI] [PubMed] [Google Scholar]