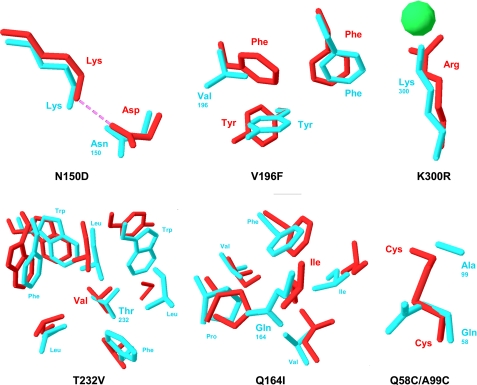
FIGURE 1.
Mutations engineered in the psychrophilic α-amylase AHA (blue) based on the structure of the mesophilic homologue PPA (red). The mutation N150D introduces a salt bridge with the corresponding Lys side chain. V196F restores a triple face-to-edge aromatic interaction. K300R provides a bidentate coordination of the chloride ion via short H-bonds as demonstrated by the crystal structure of the single mutant (43). T232V and Q164I increase the apolarity of hydrophobic core clusters (only AHA side chains are labeled for clarity), and the double mutation Q58C/A99C creates a disulfide bond. Protein Data Bank coordinate for AHA is 1AQH and for PPA is 1PPI.