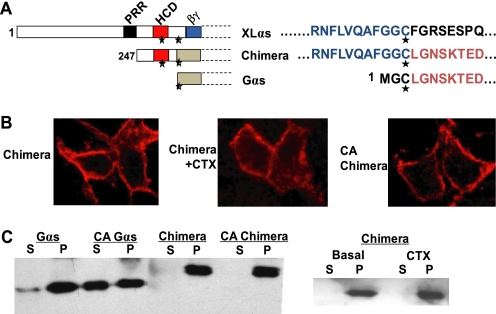
FIGURE 5.
A 72-amino acid segment of XLαs containing HCD and the two conserved cysteines is sufficient to anchor activated Gαs to the plasma membrane. A, diagram of the XLαs-Gαs chimera protein, with the 72-amino acid segment of XLαs spanning the two cysteines to replace the N-terminal 2 residues of Gαs. PRR, proline-rich region; HCD, highly charged domain; βγ, putative Gβγ interaction domain. Asterisks indicate the conserved cysteine residues. B, immunocytochemical analysis using the anti-HA antibody was performed to study the subcellular distribution of the XLαs-Gαs chimera in HEK293 cells transfected with cDNA encoding HA-tagged XLαs-Gαs chimera or a constitutively active (CA) form of the chimera carrying a GTPase inhibiting mutant analogous to R201H. CTX was also used to stimulate the native form of the chimera. C, Western blot analysis using the anti-HA antibody for comparing the subcellular localizations of Gαs, the XLαs-Gαs chimera, and the GTPase-deficient form of the chimera transiently expressed in HEK293 cells. S, soluble fraction; P, particular fraction.