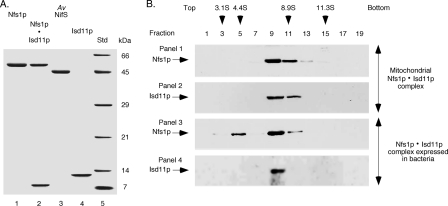
FIGURE 1.
Analysis of proteins expressed in bacteria. A, Nfs1p-His6 by itself or together with Isd11p (Nfs1p·Isd11p), A. vinelandii (Av) NifS-His6, and Isd11p-His6 were expressed in bacteria and purified by Ni-NTA chromatography. Samples were analyzed by SDS-PAGE under reducing conditions followed by Coomassie Blue staining. The difference in migration of Isd11p in lanes 2 and 4 is due to the absence or presence of the His6 tag, respectively. The molecular mass of the protein standards (Std) is indicated in kDa. B, mitochondrial extracts (panels 1 and 2) and the bacterially expressed and purified Nfs1p·Isd11p complex (panels 3 and 4) were examined by sucrose density gradient centrifugation. Alternate fractions were analyzed by SDS-PAGE under reducing conditions, followed by immunoblotting with anti-Nfs1p (panels 1 and 3) and anti-Isd11p (panels 2 and 4) antibodies. Protein standards used for the sucrose gradient centrifugation were carbonic anhydrase (3.1S), bovine serum albumin (4.4S), β-amylase (8.9S) and catalase (11.3S). The arrowheads indicate peak fractions for standards as judged by SDS-PAGE followed by Coomassie Blue staining.