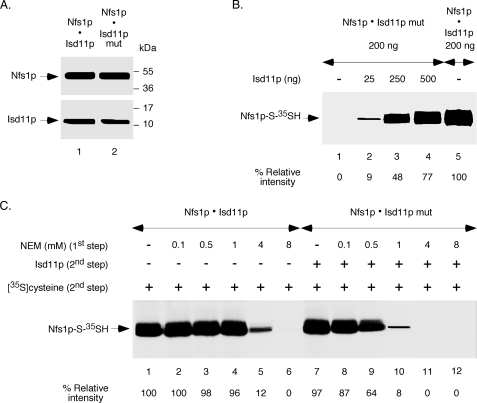
FIGURE 6.
Effects of an Isd11p mutation on interaction with Nfs1p, enzyme activation, and active site cysteine protection. A, the tripeptide 15LYK17 of Isd11p was mutated to 15AAA17, and the corresponding protein (Isd11p mut) was coexpressed with Nfs1p-His6 in bacteria in a soluble form. The Nfs1p·Isd11p mut complex was purified by Ni-NTA chromatography. Similar amounts (20 ng each) of the two complexes (Nfs1p·Isd11p and Nfs1p·Isd11p mut) were analyzed by SDS-PAGE under reducing conditions followed by immunoblotting using anti-Nfs1p and anti-Isd11p antibodies. B, the Nfs1p·Isd11p mut complex (200 ng) was incubated with PLP (150 μm) and [35S]cysteine (5 μCi) for 10 min at 30 °C in the absence or presence of increasing amounts of Isd11p. The Nfs1p·Isd11p complex (200 ng) without any added Isd11p (lane 5) served as positive control. Proteins were precipitated, and samples were analyzed by non-reducing SDS-PAGE followed by autoradiography. C, the Nfs1p·Isd11p and Nfs1p·Isd11p mut complexes (200 ng each) were treated with increasing concentrations of NEM for 10 min at 30 °C. Excess DTT (10 mm) was added to neutralize free NEM, and the protein samples obtained after ammonium sulfate precipitation were solubilized with buffer A. Isd11p (100 ng) was then added only to Nfs1p·Isd11p mut samples. All reaction mixtures were subsequently supplemented with PLP (150 μm) and [35S]cysteine (5 μCi). Samples were analyzed after incubation at 30 °C for 10 min. The intensity of Nfs1p-S-35SH for NEM-untreated Nfs1p·Isd11p (lane 1) was considered 100%.