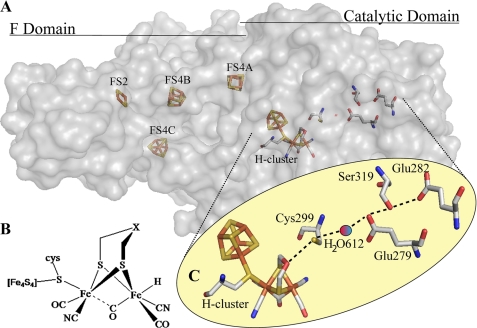
FIGURE 1.
[FeFe]-hydrogenase structure and putative proton transfer pathway. A, crystal structure of the [FeFe]-hydrogenase from C. pasteurianum (Protein Data Bank code 3C8Y) resolved to 1.39 Å. The protein is a monomer separated into a catalytic domain containing the [6Fe6S] H-cluster and three N-terminal domains that in total contain three [4Fe4S] clusters and a [2Fe2S] cluster. B, chemical structure of the H-cluster emphasizing the diiron active site. X is generally postulated to be nitrogen, although oxygen and carbon remain possibilities. C, putative proton transfer relay inferred by multiple sequence alignment analysis and examination of the crystal structure of the [FeFe]-hydrogenase from C. pasteurianum. H-cluster → Cys-299 → H2O-612 → Glu-279 → Ser-319 → Glu-282. All distances are in angstroms and are measured between oxygen and sulfur atoms.