FIGURE 9.
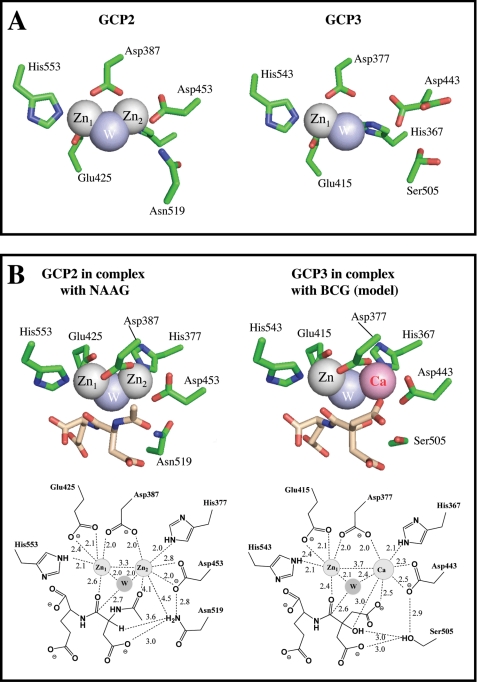
Structure of the catalytic site of GCP2 and GCP3. Panel A shows the metal cluster of the GCP2 ligand-free structure (PDB 2OOT) and of GCP3 in complex with glutamate (not depicted, PDB 3FF3). For the sake of clarity and to emphasize its low occupancy, Zn2 is not depicted in the GCP3 structure. Noteworthy, Asp-443 which coordinates Zn2 in the GCP3 structure occupies two positions, only one of which allows coordination with the Zn2 atom, in good agreement with the lower occupancy of this metal. Ser-509 also displays two conformations. In marked contrast, the Zn2 atom occupancy is 100% in the ligand-free GCP2 structure and no rotamers are observed within the amino acids constituting the catalytic site in this case. Panel B shows GCP2 in complex with NAAG (PDB 3BXM) and a modeling of GCP3 complexed with BCG and the Zn-Ca cluster. The distances between the two metals are the actual distances observed by x-ray crystallography (20). In GCP2 structures the two zinc atoms are separated by 3.3 Å, each Zn being equally distant from the catalytic water molecule (2.0 Å), whereas in GCP3 the distances between the two metals is 3.7 Å, and the distance between Me2 and the catalytic water (2.4 Å) is larger than the distance between Zn1 and this water (2.1 Å), in agreement with a bulkier calcium occupying the position of Me2.