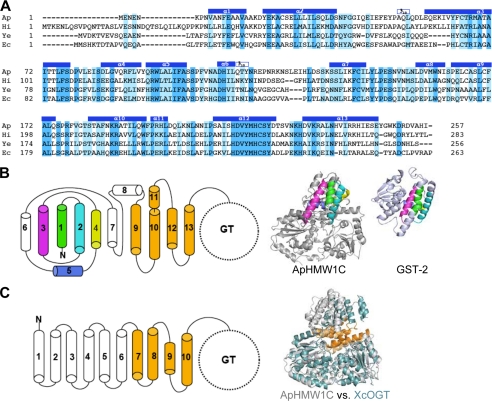
FIGURE 2.
Structural features of the AAD. A, structure-based sequence alignment of the N-terminal domain (AAD) of representative HMW1C-like proteins highlights the conserved feature of the AAD among HMW1C-like proteins. The identical and conserved residues are highlighted in blue and light blue, respectively. The helices observed in the ApHMW1C structure are indicated above the sequence: Ap, A. pleuropneumoniae HMW1C; Hi, H. influenzae HMW1C; Ec, Escherichia coli EtpC; and Ye, Yersinia enterocolitica RcsC. B, topology of the AAD in ApHMW1C. Shown are α-helices of the AAD in cylinders. The α1 to α5 helices are highlighted in green, cyan, magenta, yellow, and blue, and the α9 to α13 helices are colored in gold. In the ribbon representations, α1 to α5 of ApHMW1C and the corresponding C-terminal domain of GST-2 (PDB 3ERF) are colored as in the topology diagram. C, topology of TPRs in XcOGT. The TPR-like (TRL) helices of XcOGT are highlighted in gold. The superimposition of ApHMW1C (gray) and XcOGT (pale blue) shows the dissimilarity of N-terminal helices and the similarity of TRL helices in the two structures.