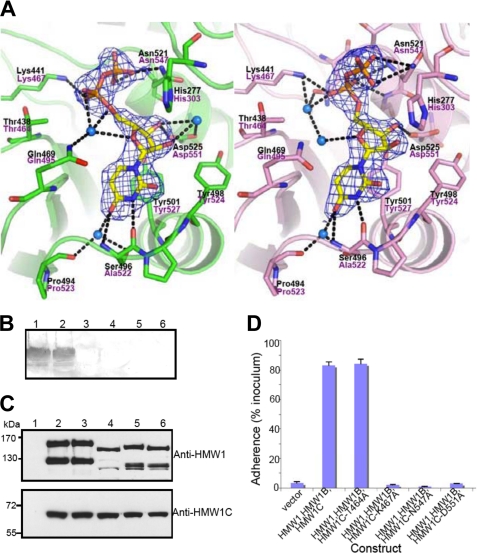
FIGURE 3.
Active site, interactions of UDP, and functional implications. A, two different conformations of UDP molecules from two protomers in the asymmetric unit are shown in stick representation with electron density (2Fo-Fc contoured at 1.3σ). Key residues involved in the interaction between ApHMW1C and UDP are indicated with side chains (stick presentation) and black letters. The corresponding residues of HMW1C are labeled in magenta letters. The protein backbone is shown with a scheme, and hydrogen-bonding interactions are indicated with black dashed lines. Water molecules mediating hydrogen bonds are shown in blue spheres. B, in vitro transferase assays examining HMW1C mutants. Assays were performed with purified HMW1802–1406, UPD-Glc, and either wild type HMW1C (lane 1), HMW1C-T464A (lane 2), HMW1C-K467A (lane 3), HMW1C-N547A (lane 4), HMW1C-D551A (lane 5), or no HMW1C (lane 6). Glycosylation was detected using DIG-Glycan reagents. C, Western immunoblots of whole cell sonicates of E. coli DH5α harboring vector only (lane 1), HMW1, HMW1B, and wild type HMW1C (lane 2), HMW1, HMW1B, and HMW1C-T464A (lane 3), HMW1, HMW1B, and HMW1C-K467A (lane 4), HMW1, HMW1B, and HMW1C-N547A (lane 5), and HMW1, HMW1B, and HMW1C-D551A (lane 6). The upper blot was performed with guinea pig antiserum GP85 against HMW1, and the lower blot was performed with guinea pig antiserum GP64 against HMW1C. D, in vitro adherence assay showing HMW1-mediated adherence by E. coli DH5α expressing HMW1 and HMW1B along with wild type H. influenzae HMW1C or an HMW1C mutant. The HWM1C residues Thr-464, Lys-467, Asn-547, and Asp-551 correspond to the ApHMW1C residues Thr-438, Lys-441, Asn-521, and Asp-525, respectively, of the binding pocket shown in A.