Fig. P1.
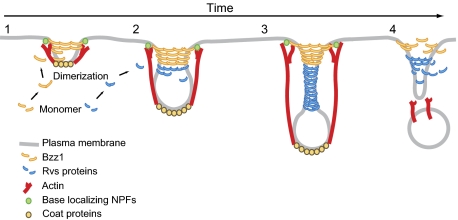
We propose that Bzz1 F-BAR protein is recruited to endocytic sites when the membrane has a relatively low curvature and assembles a rigid invagination base (step 1). Bzz1 binds to another protein, Las17, and we propose that these proteins form a stable base that resists actin assembly, enabling efficient actin assembly-driven endocytic tubule extension and constriction, which, in turn, generate higher membrane curvature (step 2). The BAR-domain proteins are then recruited to these more highly curved membrane tubules, working cooperatively with actin assembly forces to create deeply invaginated endocytic membranes, resulting in further tubule constriction (step 3) and, ultimately, in scission (step 4).