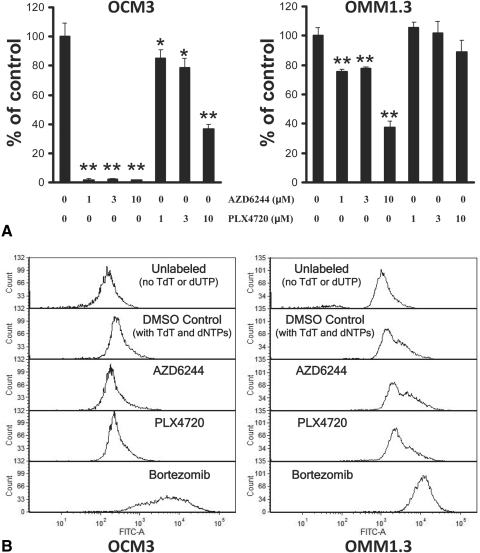
Figure 3.
Growth arrest without apoptosis in UM cell lines treated with AZD6244 or PLX4720 monotherapy. (A) Using the BrdU cell proliferation assay, we studied the impact of AZD6244 and PLX4720 on UM DNA synthesis (S phase). The BRAF-mutant OCM3 cells and the Gα-mutant OMM1.3 cells were treated with AZD6244 or PLX4720 (or DMSO) at the indicated concentrations in 24-well plates for 48 hours. Subsequently, BrdU was added to the wells for an additional 24 hours, and the amount of incorporation into DNA was quantified according to the instructions of the manufacturer and was expressed as percentage of control (DMSO) wells (average ± SD). We found that the BRAF-mutant OCM3 cells were sensitive to AZD6244 and PLX4720, whereas Gα-mutant OMM1.3 cells still responded with decreased DNA synthesis to AZD6244 (but to a lesser degree than OCM3 cells) though they were resistant to the anticancer effect of PLX4720 (*P < 0.05, **P < 0.001 vs. respective DMSO controls). (B) Using the TUNEL assay, we studied the impact of AZD6244 and PLX4720 on UM cell apoptosis. UM cells were treated with AZD6244 or PLX4720 at 3 μM (or DMSO control) for 72 hours and then labeled for apoptosis using the enzyme terminal deoxynucleotidyl transferase (TdT) and the fluorescein-dUTP. We did not detect any shift in fluorescence (TUNEL labeling) in OCM3 or OMM1.3 cells treated with AZD6244 or PLX4720 monotherapy, suggesting a lack of apoptosis. The proteasome inhibitor bortezomib (100 nM for 72 hours) served as a positive control for the induction of apoptosis. Cells incubated without TdT or fluorescein-dUTP served as a negative control.