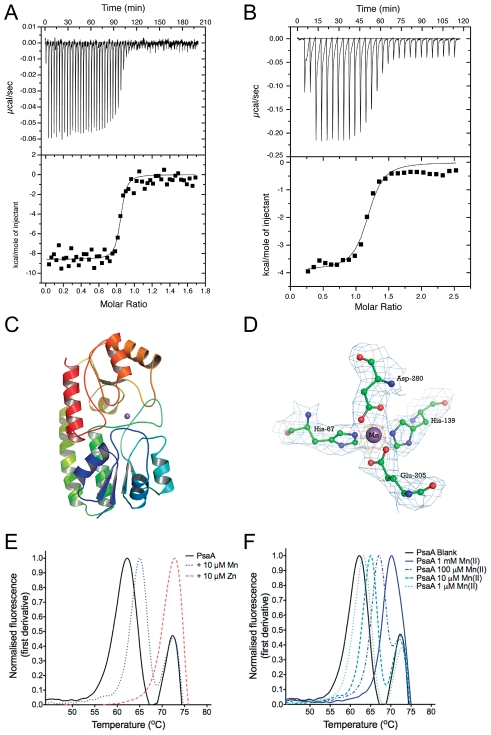
Figure 1. Biophysical characterization of purified PsaA.
(A) Representative ITC measurements for titration of 4.5 µM PsaA with 40 µM Mn(II). (B) Titration of 20 µM PsaA with 250 µM Zn(II). For each experiment the rates of heat release are shown above the corresponding plots of integrated heat. Both of the curves were fitted to a single site (n = 1) model and the KD calculated from replicate experiments (± SEM). (C) The overall fold of PsaA with the metal ion shown in purple between the two domains. (D) The metal binding site. The 2FO-Fc electron density map (contoured at the 1.0σ level) is shown in blue for the coordinating residues and the metal ion. The Mn(II) is shown as a purple sphere and the residues are in ball-and-stick representation (carbon atoms in green, oxygen in red and nitrogen in blue). Also shown in orange is the anomalous difference Fourier map contoured at the 5.0 σ level, computed using the Bijvoet differences collected at the manganese K-edge peak wavelength. (E) Thermal stability of PsaA. The sets of curves show the thermal transition of 10 µM PsaA incubated with 10 µM Mn(II) or 10 µM Zn(II). The curves are representative of three independent experiments (n = 3). (F) Thermal stability of PsaA with increasing Mn(II) concentrations as indicated.