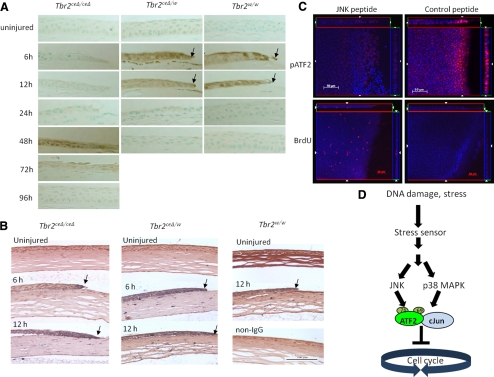
Figure 4.
Activation of ATF2 by JNK in the absence of Tbr2. Corneal epithelial debridement was performed with Tbr2ceΔ/ceΔ, Tbr2ceΔ/w, and Tbr2w/w mice, was allowed to heal for various times, and was examined by immunohistochemistry. (A, B) There was a delay of p38MAPK activation (A) in corneas of Tbr2ceΔ/ceΔ mice in comparison with the controls, but the activation of ATF2 was unaffected by the loss of Tbr2 (B). (C) To demonstrate that JNK may lead to the activation of ATF2 in the absence of Tbr2, corneas were treated with a JNK peptide inhibitor after epithelial debridement on Tbr2ceΔ/ceΔ mice and were examined for BrdU incorporation and ATF2 activation. In the presence of the JNK peptide inhibitor, ATF2 was not activated and cell proliferation was no longer suppressed 6 hours after wounding. (D) Stress from tissue injury triggered the activation of ATF2 by the phosphorylation of T69 and T71 residues by JNK and p38MAPK. Phospho-ATF2 can dimerize with itself or with other AP-1 family members, such as c-Jun. The activated ATF2-AP-1 complex blocked the cell cycle at the G1/S transition point.