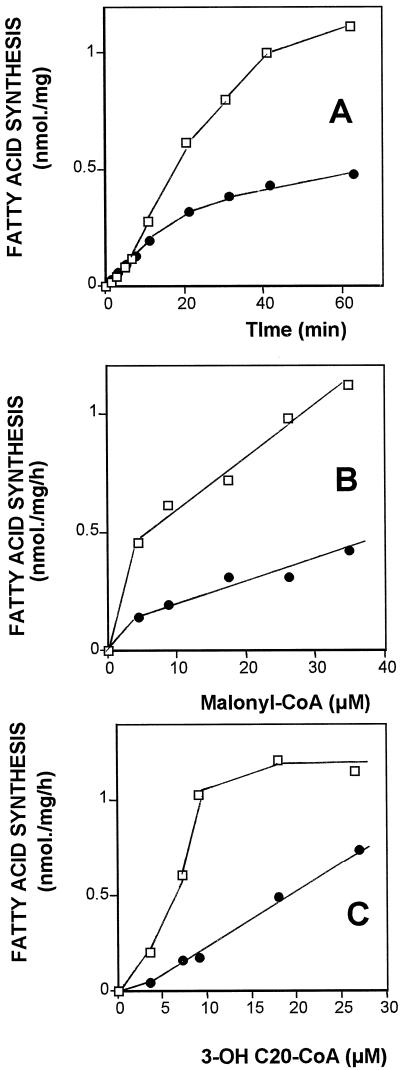
Figure 3.
Kinetic studies of (R)- and (S)-3-OH eicosanoyl-CoA isomer elongation. The (R)- (□) and (S)- (•) 3-OH-C20:0 (9 μm) elongations were measured using initial velocity conditions. A, Time-course experiment; each isomer was incubated for various times in the presence of 20 μg of protein at 30°C, with 100 μm NADPH, 1 mm MgCl2, 2 mm DTT, 0.15 mm Triton X-100, and 9 μm [2-14C]malonyl-CoA in 0.08 m Hepes buffer, pH 6.8. The reaction was stopped after different incubation times and the radioactivity was measured in the fatty acid fraction as indicated in Methods. B, Effect of malonyl-CoA concentration; 20 μg of protein was incubated for 20 min; the malonyl-CoA concentrations are indicated. C, Effect of 3-OH acyl-CoA concentration; same conditions as in A. Incubation time was 20 min, and acyl-CoA concentration varied as indicated.