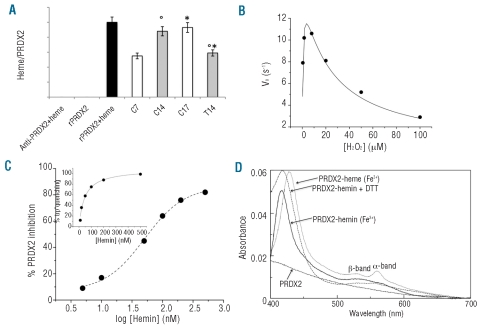
Figure 5.
Recovery of heme bound to immunoprecipitated PRDX2 and functional features of recombinant PRDX2 in the presence or absence of heme. (A) Heme bound to immunoprecipitated PRDX2 is expressed as a ratio between the heme levels measured as reported in the Design and Methods section and the amount of PRDX2 immunoprecipitated and evaluated by densitometric analyses. The heme levels were measured in the following samples: column 1: anti-peroxiredoxin-2 antibody (Ab) incubated with heme; column 2: recombinant PRDX2 alone; column 3: recombinant PRDX2 previously bound to heme and then immunoprecipitated (Ab) with specific anti-PRDX2 antibody (black); columns 4–7 immunoprecipitated PRDX2 from normal controls (C) and β-thalassemic (T) erythroid precursors at the different time points studied C7 and T7 (white bars, 7 days of culture), C14 and T14 (gray bars, 14 days of culture). Errors were reported as the S.E.M. as bars. *compared to control cells; °comparison of 14 versus 7 days. (B) Kinetic parameters of recombinant PRDX2 in the presence of increasing concentrations of H2O2. The data points were fitted to equation 1 which accounts for substrate inhibition. Errors were less than 10%. (C) Inhibitory effect of increasing concentrations of hemin on recombinant PRDX2 activity in the presence of saturating concentrations of H2O2. The IC50 was determined by fitting inhibition data to a dose-response sigmoidal curve. The inset shows the binding of hemin to PRDX2 determined by following tryptophan emission quenching after the addition of increasing concentrations of hemin. Data were fitted to equation 2. (D) The absorption spectra of the PRDX2-heme complex measured in the presence of: oxidized (Fe3+) (−), reduced (Fe2+) (…) and 1 mM DTT (-.-.). Equimolar amounts of PRDX2 and hemin were mixed and UV-visual spectra recorded in 50 mM Hepes pH 7.5 at room temperature. Heme bound to recombinant PRDX2 was reduced with a 1000-fold molar excess of sodium dithionite. The visible spectrum of unbound PRDX2 is reported (- - -).