Abstract
Background
Despite extensive investigation, the cause of liver injury in 14% of acute liver failure patients remains unknown (indeterminate). In a pilot study, using a novel assay, highly specific acetaminophen-cysteine adducts were detected in 7 of 36 (19%) indeterminate patients.
Methods
To extend these observations, sera from 110 subjects enrolled in the Acute Liver Failure Study Group registry with indeterminate acute liver failure were analyzed using a similar but more efficient and sensitive adduct assay. As positive controls, an additional 199 patients with known or presumed acetaminophen-induced liver failure were assessed for the presence and quantity of adducts. Clinical, laboratory and outcome data were compared for the two groups.
Results
Based on previous data from known therapeutic exposures and acetaminophen overdoses, an adduct concentration of ≥1.0 nmol/mL serum indicated a definite acetaminophen overdose. Among the 110 indeterminate cases, 18% had assay values ≥1.0, with a median level of 9.2 nmol/mL; 94.5 % of the positive control (known APAP) cases had values ≥1.0 nmol/mL. Regardless of initial diagnosis, subjects with elevated adduct levels demonstrated the clinical profile and hyperacute biochemical injury pattern associated with acetaminophen overdose: predominance of female gender, very high aminotransferase levels and low bilirubin levels.
Conclusions
These data confirm and extend previous observations regarding the high (18%) prevalence of unrecognized or uncertain acetaminophen toxicity among subjects with indeterminate acute liver failure. N-acetylcysteine use was limited in this group, presumably because of the lack of a specific diagnosis of acetaminophen toxicity.
Keywords: APAP, ALF, adduct, NAC
Introduction
Acute liver failure (ALF) is a rare but life-threatening condition occurring in individuals without pre-existing liver disease and is characterized by sudden severe liver dysfunction associated with coagulopathy (international normalized ratio >1.5) and hepatic encephalopathy.1,2 Over the last decade, clinically defined acetaminophen (APAP; a dose-dependent hepatotoxin), overdose has been the most common cause of adult ALF in the United States, accounting for close to 50% of all cases.2 The next most frequent cause of ALF, accounting for approximately 14% of the total, is comprised of those individuals for whom an identifiable etiology cannot be found.3 These indeterminate cases are classified as such after extensive evaluation, including medical history, physical examination, laboratory testing and, specifically, the exclusion of clinically defined APAP overdose patients.4
Low doses of APAP are primarily metabolized by glucuronidation and sulfation. With higher doses, the conjugation pathways become saturated and metabolism of APAP is shunted to the cytochrome P450 system generating the highly reactive toxic metabolite, N-acetyl-p-benzoquinoneimine (NAPQI).5 NAPQI binds to hepatocellular proteins when glutathione stores, normally involved in detoxification, are depleted.6 The resulting APAP-CYS protein adducts (APAP bound to cellular proteins via cysteine residues) can be quantified by a recently-developed assay employing high-pressure liquid chromatography with electrochemical detection (HPLC-EC).7 Previous data in the mouse model of APAP toxicity using immunohistochemical approaches have demonstrated that adducts localize in the centrilobular hepatocytes of the liver and that these same cells subsequently lyse, releasing both adducts and hepatic transaminases in the serum.8,9
Although the HPLC-EC assay is not yet available for clinical use, it allows for the detection of a highly specific biomarker of APAP hepatotoxicity in occult or late presentation ALF cases, when the parent compound is at low or undetectable levels in the plasma.6 In the initial study, samples were analyzed in blinded fashion from 81 patients: 36 with indeterminate ALF, 20 certain acetaminophen cases and 25 other disease controls for which there was no history of acetaminophen exposure. The assay correctly identified all cases of acetaminophen toxicity and was negative in all patients with ALF due to other causes. In addition, adducts were detected in 7 (19%) of the 36 indeterminate cases.6. Adducts have also been identified in 12.5% of pediatric patients with ALF of indeterminate etiology,10 and may be detected for up to 12 days after clinically defined APAP overdose.11,12
To confirm and extend our previous report regarding the detection of adducts in patients with ALF of indeterminate etiology,6 the following study was conducted in a larger cohort of patients with ALF.
Methods
Study Population
The US Acute Liver Failure Study Group (ALFSG) was established in 1998 as a consortium of liver centers interested in better defining the causes and outcomes of acute liver failure. To date, 1,431 subjects have been enrolled prospectively at 23 tertiary centers within the US, all of which have liver transplantation programs. All enrolled subjects met standard criteria for acute liver failure: presence of coagulopathy (prothrombin time >15 seconds or international normalized ratio ≥1.5) and any degree of hepatic encephalopathy, occurring within 26 weeks of the onset of first symptoms in a patient without previous underlying liver disease.1,3 Since the subjects were encephalopathic by definition, written informed consent was obtained from their legal next of kin. Detailed demographic, clinical, laboratory and outcome data as well as daily sera for 7 days, a DNA sample and tissue (when available) were collected prospectively. All centers were in compliance with their local institutional review board requirements. A Certificate of Confidentiality was obtained from the National Institutes for Mental Health for the entire study.
Assessment of indeterminate and APAP-induced ALF
Each site’s principal investigator, an academic hepatologist, was responsible for ascertaining the etiology of the ALF by careful historical review and extensive clinical, radiographic, laboratory and pathological evaluation as needed using standard criteria.3 The etiology was considered indeterminate when the above evaluations failed to indicate a defined cause, specifically when no clear evidence for clinically defined APAP overdose was found. During the period between 1998 and 2006, 1,115 patients were enrolled, of whom 158 were considered to have indeterminate ALF.
For all patients with any suspicion of APAP ingestion, a careful history of the ingestion was obtained, including the total dose ingested, specific APAP product taken and duration of use. For the 275 APAP cases that were included in the previous paper describing the clinical features of acetaminophen toxicity, an additional audit was performed by one investigator (AML).2 Criteria used in the previously published paper for assigning acetaminophen as the cause of ALF were: (1) a history of potentially toxic acetaminophen ingestion (i.e., greater than 4 g APAP per day which is the maximum dose approved for use by the FDA on the package) within 7 days of presentation; (2) detection of any level of acetaminophen in the serum; or (3) a serum alanine aminotransferase (ALT) ≥1,000 IU/L with a history of acetaminophen ingestion, irrespective of the acetaminophen level.
Severity markers
Hepatic coma was graded on a standard scale of I to IV.3 The Model for End Stage Liver Disease (MELD) score and the King’s College Hospital Criteria for ALF (“King’s Criteria”) were used to assess overall severity of illness upon enrollment.13,14 For MELD scores, etiology was assumed to be non-alcoholic and non-cholestatic, lab tests less than 1.0 were corrected to 1.0, and serum creatinine values were assumed to be 4.0 mg/dL for all values >4.0 mg/dL, or if the patient received dialysis, as described elsewhere.15 Outcomes (survival, death, transplantation) were determined 3 weeks after study admission using a standardized case report form. Spontaneous survival was defined as those alive at three weeks without transplantation. Overall survival was defined as those who survived at 3 weeks regardless of transplantation status. Patient management was based on local standard of care, although general guidelines were employed by the ALFSG sites.4 Liver transplant candidacy was determined at individual centers along United Network of Organ Sharing guidelines.16
Measurement of serum APAP-adduct concentrations
From the 1,115 cases in the registry, we identified 158 (14%) patients as having indeterminate ALF. Sera from study day 1 or 2 were available in 110 subjects (69.6%) (Figure 1: Groups 1 and 2). From our previous clinically defined APAP overdose patient cohort, sera were available from study day 1 or 2 for 199 of 275 (72.4%)2 (Figure 1: Groups 3 and 4). Sera collected at each site had been aliquoted promptly and stored at -80°C, shipped to a central storage site, then to the analytical laboratory of one investigator (LPJ). Serum samples (100-500 μL each) from 309 subjects (110 + 199, Figure 1) were analyzed in a blinded fashion for acetaminophen protein adducts using a previously reported method.7,12 In brief, serum samples were treated with gel filtration to remove small molecules including acetaminophen and acetaminophen metabolites. The resulting sample was treated with protease and the resulting peptides analyzed for APAP-CYS adducts by HPLC-EC.7,12 The range of linearity for the method was 0.03 to 30.0 nmol APAP-CYS adducts per mL of serum. The coefficients of variation (CVs) for the assay were consistently <15% at concentrations of 0.03, 1.0, 6.0 and 30.0 nmol/mL APAP-CYS adducts. Based on the CVs for the standard curve for the assay, the lower limit of quantitation was defined as 0.03 μM APAP-CYS. In a recent study, a receiver operator curve analysis found that a cut point of ≥1.1 nmol adducts/mL serum in patients with clinically defined APAP overdose and an ALT value >1000 IU/L had a sensitivity of 96.8% and a specificity of 95%.12
Figure 1.
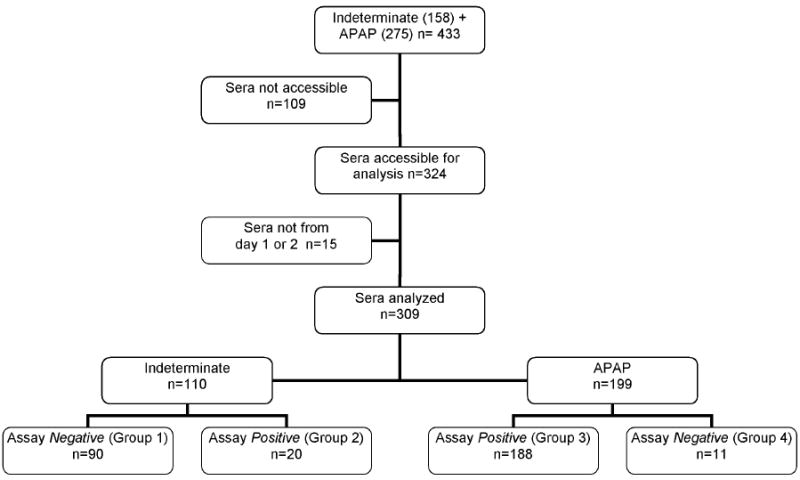
Flow diagram of testing for patients diagnosed initially with either Indeterminate or APAP ALF with assay results
Statistical methods
Using the standard guidelines and appropriate controls established for the assay, we compared differences in clinical characteristics in subjects with negative and positive levels of APAP-adducts. ‘Positive’ was used to designate samples having levels of ≥ 1 nmol/mL while ‘negative’ was used to designate samples having adduct levels < 1 nmol/mL. To compare continuous variables among the groups, the one-way analysis of variance (ANOVA) was used unless the Bartlett’s test showed unequal variances, then the Kruskal-Wallis test was used. To compare categorical variables among the groups, χ2 or Fisher’s exact test was used. Once a global p-value was ascertained, indicating that a difference existed, comparisons were made between two groups using a student t, Mann-Whitney U, or χ2 test where appropriate. For all analyses, a two-tailed p-value of ≤0.05 was considered significant, and adjusted for multiple two-way comparisons.
Results
Study subjects
Overall results of the APAP-CYS adduct assay are presented in Figure 1 and Table 1. Of 110 subjects with indeterminate ALF, 20 (18%) had levels of APAP-CYS adducts suggesting that the ALF was secondary to an acetaminophen overdose. For the purposes of the present study, results of adduct assays ≥ 1.0 were defined as positive, while adduct assay results < 1.0 nmol/mL were defined as negative, signifying that the adduct values were below levels previously identified as being associated with clinically defined APAP overdose and development of an ALT > 1000 IU/L.12 Using the previously defined cutoff, the overall indeterminate group was divided into the 90 < 1.0 nmol/mL (Group 1) and the remaining 20 demonstrating significant quantities of APAP adducts (Group 2). Among those in Group 2, the median APAP-CYS concentration was 9.2 nmol/mL as compared with 0.0 nmol/mL in Group 1, all of whose values were below threshold (Table 1). Of the 199 clinically defined APAP overdose patients’ sera analyzed, 188 had values above the threshold for a toxic exposure (Group 3), while 11 were below the toxicity threshold (Group 4). Thus, 94.5% of the patients with clinically defined APAP overdose had APAP-CYS concentrations that were above the threshold for toxicity. The median APAP-CYS concentration was 11.1 nmol/mL for the 188 APAP-CYS positive patients (Group 3) vs. 0.3 nmol/mL for the 11 APAP-CYS negative patients (Group 4).
TABLE 1.
Baseline Features in Indeterminate ALF compared to clinically defined APAP overdose ALF, stratified by Assay Status
| Characteristic | (Group 1) Indeterminate: Assay Negative n=90 | (Group 2) Indeterminate: Assay Positive* n=20 | (Group 3) APAP: Assay Positive* n=188 | (Group 4) APAP: Assay Negative n=11 | Global p-value | Group Comparison¶ |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years | ||||||
| Median | 39 | 32.5 | 37 | 42 | <0.025 | NS |
| 25th %, 75th %-tile | 27, 51 | 23.5, 38.5 | 30, 45 | 37, 43 | ||
| Female [n (%)] | 43 (47.8) | 16 (80.0) | 140 (74.5) | 6 (54.5) | <0.001 | 1 vs 3 |
| Laboratory Markers | ||||||
| ALT, IU/L | ||||||
| Median | 811 | 5156 | 4025 | 1810 | <0.001 | 1 vs 2 |
| 25th %, 75th %-tile | 335, 1405 | 3463, 8140 | 2422, 6680 | 621, 9386 | 1 vs 3 | |
| AST, IU/L | ||||||
| Median | 691 | 8126 | 4333 | 1808 | <0.001 | 1 vs 2 |
| 25th %, 75th %-tile | 342, 1314 | 5162, 12295 | 2027, 8192 | 277, 10860 | 1 vs 3 | |
| Bilirubin, mg/dL | 1 vs 2 | |||||
| Median | 24.3 | 5.05 | 4.1 | 7.3 | <0.001 | 1 vs 3 |
| 25th %, 75th %-tile | 17.6, 31.7 | 2.5, 6.5 | 3.0, 5.9 | 1.4, 11.1 | 1 vs 4 | |
| APAP-Assay, nmol/mL | <0.001 | 1 vs 2 | ||||
| Median | 0 | 9.2 | 11.1 | 0.25 | 1 vs 3 | |
| 25th %, 75th %-tile | 0, 0.4 | 4.4, 14.3 | 6.7, 18.6 | 0.2, 0.3 | 1 vs 4 | |
| 2 vs 4 | ||||||
| 3 vs 4 | ||||||
| Severity Markers | ||||||
| MELD score | ||||||
| Mean | 35.5 | 34.6 | 31.7 | 33.7 | 0.014 | 1 vs 3 |
| SD | 9.2 | 6.8 | 9.6 | 11.0 | ||
| APAP King’s | ||||||
| Criteria met [n (%)] | 9 (10.0) | 5 (25.0) | 29 (15.4) | 1 (9.1) | NS | |
| Hepatic Coma | ||||||
| Grade (1-4) [n (%)] | ||||||
| 1 | 19 (22.6) | 3 (16.7) | 46 (25.3) | 2 (20.0) | NS | |
| 2 | 26 (31.0) | 3 (16.7) | 43 (23.6) | 1 (10.0) | ||
| 3 | 14 (16.7) | 6 (33.3) | 42 (23.1) | 4 (40.0) | ||
| 4 | 25 (29.8) | 6 (33.3) | 51 (28.0) | 3 (30.0) | ||
| Any NAC Treatment [n (%)] | 16 (17.8) | 8 (40.0) | 177 (94.1) | 9 (81.8) | <0.001 | 1 vs 3 |
| 1 vs 4 | ||||||
| 2 vs 3 | ||||||
| Outcomes | ||||||
| Spontaneous survival [n (%)] | 19 (21.1) | 11 (55.0) | 120 (63.8) | 5 (45.5) | <0.001 | 1 vs 2 |
| 1 vs 3 | ||||||
| Liver transplantation [n (%)] | 39 ( 42.2) | 2 (10.0) | 15 (8.0) | 2 (18.2) | <0.001 | 1 vs 2 |
| 1 vs 3 |
Assay positive defined by ≥ 1.0 nmol/ml; NS = not-significant (p>0.05)
Statistically significant two-way Group comparisons shown with adjusted p-values < 0.05; where adjusted p-value defined as actual p-value x number of comparisons made (6)
Baseline Characteristics
Comparison of the four groups
The 20 APAP-CYS positive indeterminate-ALF patients (Group 2) were remarkably similar to the 188 APAP-CYS positive control patients (Group 3) in demographic, clinical and laboratory markers of disease. The APAP-CYS positive patients were younger than their APAP-CYS negative counterparts for both the indeterminate cohort (Group 1 median age 32.5 years vs. Group 2, 39 years) and the APAP control cohort (Group 3 median age 37 vs. Group 4, 42 years), though these differences were not statistically significant. The APAP-CYS positive patients were predominantly female (Group 2 80%, Group 3 74.5%), compared with their APAP-CYS negative counterparts for both the indeterminate cohort 48% (Group 1) and control cohort 54.5% (Group 4). Groups 2 and 3 had median ALT values >4,000 IU/L (4,025 and 5,156 IU/L, respectively); whereas the median ALT for Group 1 was much lower (811 IU/L) (p<0.05). The same was true for aspartate aminotransferase (AST) values with very high levels of AST observed in Groups 2 and 3 and significantly lower ones in Group 1. Bilirubin concentrations were much lower in both APAP-CYS positive groups (Group 2, median 5.05 mg/dL and Group 3, 4.1 mg/dl), as compared to Group 1: median 24.3 mg/dL (p<0.05). Group 4, the APAP-CYS negative control group, also had a lower median bilirubin concentration (7.3 mg/dL, p<0.05) compared to Group 1 and similar to Groups 2 and 3. Of the 20 APAP-CYS positive indeterminates (Group 2), 19 (95%) had an ALT >1,000 IU/L (17 (85%) had ALT >3,000 IU/L) and 18 (90%) had a bilirubin <10 mg/dL. Similarly, of the 188 APAP-CYS positive controls (Group 3), 175 (93%) had an ALT >1,000 IU/L (117 (62%) had ALT >3,000 IU/L) and 175 (93%) had bilirubin levels of <10 mg/dL.
Retrospective review of the 20 adduct positive indeterminate cases disclosed that the circumstantial evidence favored acetaminophen in some cases. Twelve of the 20 patients had this evidence suggesting APAP as the cause, hence they were considered ill-defined APAP overdose patients by the site principal investigator (PI). In hindsight, clinically defined APAP overdose seemed a possible or even likely diagnosis. In each of these cases, there were high aminotransferases (all > 1,500 IU/L) and either a low but measurable acetaminophen level or a history of taking some acetaminophen containing medications. Of interest, all but 2 of the 12 were graded as coma grade II or higher. Of the 8 remaining patients, 7 had negative APAP levels; in 5 patients there was no history supporting any acetaminophen ingestion. Thus, these 8 were clinically unrecognized APAP patients. In 2 of the 8, there was retrospective support for an APAP overdose (previous APAP overdose but denied by patient and a report of APAP overdose in an autopsy report but not in the case report form).
Retrospective review of the 11 patients considered to be acetaminophen overdose cases but not confirmed by elevated adduct levels (Group 4), disclosed that in 6 cases there was late presentation to the medical facility ranging from 5 to 14 days following ingestion or onset of symptoms. Of these 6 late presenters, 3 patients had low ALT levels less than 1,000 IU/L, while 1 out of the 5 early presenters had low ALT levels. In the remaining 4, 3 had high aminotransferases, strong histories, and measurable or high acetaminophen levels, while the final patient was probably diagnosed in error on the basis of high enzyme levels. He had no obtainable history and had a known seizure disorder but had been found unconscious. There was no APAP level, aminotransferases were above 3,000 IU/L (AST 10,860 IU/L and ALT 3,779 IU/L) and more likely was suffering from status epilepticus with ischemic injury and myonecrosis. Repeat adduct analysis was performed in these 11 cases using separate serum samples and all repeat assays were < 1.0 nmol/ml.
The clinical and laboratory data of the 48 indeterminate patients that were excluded because their sera were not available for APAP-CYS adduct testing were analyzed and resembled the remaining Group 1 patients. Briefly, 67% were female, and the median age was 34 years and biochemical profile showed a high median bilirubin concentration (24.6 mg/dL) and a low median levels for AST (780 IU/L) and ALT (649 IU/L).
Severity Markers and Clinical Outcomes
Further analysis was conducted to compare physiologic scores and patient outcomes between the four groups. The average Model for End Stage Liver Disease (MELD) score, an established marker of liver failure severity, was statistically similar between Groups 2 and 3 (p=0.56). Hepatic coma grade on admission and the percentage of patients who met King’s criteria were similar across all four groups. Outcomes were similar between the two APAP-CYS positive groups (Table 1), with similar short-term spontaneous survival rates (55 vs. 63.8%) and liver transplantation rates (10 vs. 8%).
Non-APAP patients, assay negative (below threshold, Group 1)
The mean MELD score for the 90 non-APAP Group 1 patients, was higher than Group 3, the clinically defined APAP overdose control group (35.5 vs. 31.7; p<0.014), largely because of the differences in bilirubin concentrations between the groups (higher in Group 1, Table 1). This difference was not due to having larger sample sizes because the standard deviations (or IQR) are larger than Group 2.
Likewise, Group 1 had a 4- to 5-fold higher liver transplantation rate at 42% compared to Group 2 or Group 3 (p < 0.05 for both comparisons). The spontaneous (transplant-free) survival rate for Group 1 was significantly lower at 21% as compared to 63 and 55% in Groups 2 and 3, respectively (p<0.05 for both comparisons).
N-acetylcysteine (NAC) treatment
The percentage of patients that received NAC differed across the four patient groups (Table 2). Patients from Group 3 with clinically defined APAP overdose received NAC 94.1% of the time compared to only 40% in the adduct-positive indeterminate (Group 2) patients. Still, the 40% NAC use for this group was considerably higher than the 17.8% use recorded for Group 1, the non-APAP or adduct-negative indeterminate group. Group 4 NAC use was 81.8%, as befits clinically defined APAP overdose patients. While 100% use of NAC would be ideal, it should be pointed out that this series represents patients developing liver failure, not the overall clinical spectrum of acetaminophen overdoses. Thus, the patients in this group represent the most severe cases, and in some cases failure of the initial treating physician to begin NAC in a timely fashion. Spontaneous survival was slightly lower in Group 2 than Group 3: 55% vs. 63.8%, but this was not significantly different. In the present patient series, 24 indeterminate patients were enrolled in a double blind, placebo-controlled trial of its use in ‘non-acetaminophen’ ALF, with 10 receiving NAC and 14 receiving placebo (Table 2). An additional 14 patients had received NAC at some point in their care, though not within the aegis of the clinical trial, since the trial excluded participation if there was prior NAC use. Prior NAC treatment outside of the trial took place in 5/14 (35.7%) of Group 2 and 9/72 of Group 1 (12.5%, Table 2); NAC-treated patients, regardless of how treatment was provided, showed roughly comparable outcomes (data not shown).
TABLE 2.
Comparison of severity markers and outcomes between the four groups, stratified by use of NAC.
| Characteristic | (Group 1) Indeterminate: Assay Negative n=90 | (Group 2) Indeterminate: Assay Positive* n=20 | (Group 3) APAP: Assay Positive* N=188 | (Group 4) APAP: Assay Negative n=11 | ||||
|---|---|---|---|---|---|---|---|---|
| Any NAC treatment | Y | N | Y | N | Y | N | Y | N |
| NAC tx: overall [n (%)] | 16 (17.8) | 74 (82.2) | 8 (40.0) | 12 (60.0) | 177 (94.1) | 11 (5.9) | 9 (81.8) | 2 (18.2) |
|
| ||||||||
| Controlled tx (within RCT) [n] | 7 | 11§ | 3 | 3§ | 0 | 0 | 0 | 0 |
| Prior NAC tx [n] | 9 | 63 | 5 | 9 | 177 | 11 | 9 | 2 |
| Severity Markers | ||||||||
| MELD score | ||||||||
| Mean | 35.9 | 35.4 | 34.6 | 34.5 | 31.6 | 32.5 | 33.2 | 35.8 |
| SD | 10.4 | 9.0 | 6.0 | 7.6 | 9.6 | 9.2 | 12.2 | 3.3 |
| ALT ≥1000 IU/L [n (%)] | 9 (56.3) | 26 (35.1) | 8 (100) | 11 (91.7) | 164 (92.7) | 11 (100) | 6 (66.7) | 1 (50.0) |
| Outcomes | ||||||||
| Spontaneous survival [n (%)] | 6 (37.5) | 13 (17.6) | 6 (75.0) | 5 (41.7) | 113 (63.8) | 7 (63.6) | 4 (44.4) | 1 (50.0) |
| Liver transplantation [n (%)] | 6 (37.5) | 33 (44.6) | 0 | 2 (16.7) | 14 (7.9) | 1 (9.1) | 2 (22.2) | 0 |
| Overall 3-wk survival [n (%)] | 11 (68.8) | 45 (60.8) | 6 (75.0) | 5 (41.7) | 122 (68.9) | 8 (72.7) | 6 (66.7) | 1 (50.0) |
Assay positive defined by ≥ 1.0 nmol/mL
Received placebo
Discussion
The present study confirms and extends the previous findings of our earlier report6 regarding the use of the acetaminophen protein adduct assay in the diagnostic evaluation of patients with acute liver failure. In this larger data set using a refined assay method, acetaminophen protein adducts were detected in 95% of patients with clinically defined APAP overdose. In addition, nearly one fifth of patients with ALF classified as indeterminate showed evidence of acetaminophen toxicity not identified by experienced investigators using current diagnostic techniques. Reasons for failing to diagnose cases include the presence of hepatic encephalopathy on admission, possible deception by patients or their failure to recognize and report excessive dosing of this readily available product. In some instances, a vague history of acetaminophen ingestion had been provided by the patient or family but could not be confirmed. The clinical features observed in the ill defined APAP overdose patients and the clinically unrecognized APAP patients (collectively referred to as Group 2) were remarkably similar in demographics, laboratory values and outcomes to the ALF cases of clinically defined APAP overdose and perhaps should have been recognized on that basis. Using the clinical characteristics as criteria for APAP toxicity in patients with unclear acetaminophen histories may allow earlier identification of these cases in the absence of an available acetaminophen adduct assay. This study was not intended to validate the previously published criteria assigning acetaminophen as the cause of ALF. Using those criteria in an emergency setting may lead to over-diagnosis of APAP-induced hepatotoxicity and, therefore, of APAP-induced ALF. However, the aim of those criteria is to be more sensitive than specific in detection of APAP-induced hepatoxicity.
Patients with unrecognized APAP hepatotoxicity received NAC treatment much less often than patients with clinically defined APAP overdose, as might be expected. This withholding of care is likely to have affected outcome had larger patient numbers been included. Withholding of NAC in the future may be less likely to occur, given recent evidence that NAC is of value in the setting of non-acetaminophen ALF.17 Recognition of acetaminophen overdosing is important for other reasons: this knowledge may impact the decision to continue NAC, decisions concerning transplant candidacy, prognosis, referral for psychiatric counseling and educational intervention for unintentional cases. Although NAC is being increasingly used for non-APAP cases, the practice is not necessarily widespread among all practitioners. Finally, having a better diagnostic test for this condition should provide clinicians with better epidemiologic data and enhance future education and prevention efforts.
The pattern of hyper-acute liver injury (low bilirubin, high ALT) was almost exclusively confined to the two adduct-positive groups, supporting the short duration, hyper-acute illness pattern associated with APAP toxicity. However, this hyper-acute liver injury pattern also occurs in ischemic liver injury following decrease in cardiac output for any reason. In the absence of a readily available adduct assay, clinicians should look for the hyperacute pattern with short duration illness, high aminotransferases and low bilirubin as likely indicating APAP toxicity (or possible ischemic hepatitis). By contrast, Group 4, those with clinically defined APAP overdose but having adducts below the toxicity threshold, had somewhat atypical APAP features with a heterogeneous demographic, clinical, biochemical, and outcome profile. Dose history was not available for six of the 11 patients and six of the 11 had some but unclear histories of ethanol use. Six of the 11 patients had “late presentations” to medical centers, more than 7 days after onset of illness. While every attempt was made to use data 1 or day 2 study samples for this analysis, approximately 30% of patients in the overall analysis had study samples that were study day 3 or greater. From the limited sample size, no clear pattern can best identify this subgroup, its prognosis, or why these 11 tested negative (or below the threshold) in the adduct assay, although clearance of adducts is possible in patients presenting late.12 Another less likely possibility is that NAC treatment could have affected the assay results, though nearly all of Group 3 with clinically defined APAP overdose had NAC treatment (95%) and adducts were positive. Further studies are needed to truly understand the impact of NAC on the interpretation of the acetaminophen adduct assay via a prospective analysis or possibly animal models. It is important to note that the previous receiver operating characteristic analysis (and the generation of sensitivity and specificity parameters) of adduct levels in patients with clinically defined APAP overdose was anchored to patients with an ALT of > 1000 IU/L. Thus, non-toxic levels of adducts or lower levels of adducts in patients with an ALT of < 1000 IU/L is not unexpected.12
The significant differences in the magnitude of acetaminophen protein adduct levels between clinically defined acetaminophen overdoses and regular therapeutic use of acetaminophen were recognized in two previous studies. We previously reported the pharmacokinetic profile of adducts in 18 adults with clinically defined APAP overdose and found the mean elimination half life to be 1.73 days.12 In this analysis, adduct values were plotted relative to the day of overdose so that adduct levels at day 3 of overdose were in the 7-9 nmol/mL range and by day 8-10 of the overdose, the levels were closer to the 1.0 nmol/mL cutoff point. In some patients, levels were < 1.0 nmol/mL at days 8-10. In a further study, adduct formation was examined in healthy adults receiving APAP 4 grams per day in an inpatient clinical study setting.18 Low levels of adducts were detected in these patients; however, the mean Cmax (maximum plasma concentration) value for adducts was 0.3 nmol/mL, a value that was approximately two orders of magnitude below the levels observed in the early stages of very serious cases of clinically defined APAP overdose. Thus, very low levels in the late stages of toxicity cannot be distinguished from levels that would be detected in patients receiving therapeutic doses, but the peak of symptoms and accompanying hepatic transaminase elevation occurs early (2-4 days) in overdose patients.
Outcomes for those unrecognized adduct positive patients who did not receive the NAC antidote were poorer (5/12, 42%) compared to those who received NAC (6/8, 75%) (Table 2); both these groups, however, were relatively small. Taken together, our results indicate that NAC improved the short-term spontaneous survival in both adduct positive and adduct negative indeterminate groups.
The present study suggests that all patients presenting with rapid onset ALF in whom an etiology cannot be determined should be considered potential acetaminophen cases. In the absence of a readily available adduct assay, clinicians should look for the hyperacute pattern with short duration illness, high aminotransferases and low bilirubin as likely indicating APAP toxicity. Rapid institution of NAC is indicated in most circumstances, since its toxicity is low and its value is well established in acetaminophen toxicity and appears useful for non-acetaminophen cases as well.
The cause of the remaining 82% of indeterminate cases remains obscure. Other studies from our group have thus far failed to identify either new viruses or toxins involved in the indeterminate group.19-22 Nevertheless, use of the adduct assay, when it becomes available, should lead to earlier detection of acetaminophen hepatotoxicity and thus facilitate more aggressive NAC treatment---use of NAC on a presumptive basis in the absence of confirmed toxicity should not be discouraged.
In conclusion, indeterminate acute liver failure accounts for ~14% of all acute liver failure referred to tertiary centers. However, this group includes nearly approximately 18% suffering from unrecognized APAP toxicity, demonstrated by the presence of high levels of APAP-CYS adducts in serum and clinical and biochemical profiles virtually identical to known APAP cases. The fact that 18% of APAP hepatoxicity was missed by experienced clinicians is of concern for point of care physicians and tertiary academic hepatologists. Lack of early recognition of APAP hepatoxicity may indicate that many physicians as well as patients are uninformed about the ubiquity of the acetaminophen problem. Because of its frequency, practitioners should consider readily the diagnosis of APAP hepatoxicity in the proper setting even when an adequate history of ingestion is unavailable. Moreover, clinicians should have a low threshold for using NAC in the setting of hyperacute liver failure, since adducts have been found in significant levels even in the absence of obvious clinically defined APAP toxicity. For the remaining 80% of indeterminate patients with no evidence of APAP hepatotoxicity, spontaneous survival is considerably less than that of APAP cases. The subacute disease pattern includes lower aminotransferase concentrations, higher bilirubin concentrations and a higher rate of transplantation; this group may benefit from NAC as well. Prompt referral of all cases of potential ALF, regardless of etiology, should be made to centers where liver transplantation is available.
TABLE 3.
Summary features for 11 Group 4 patients; APAP adduct assay negative.
| 1 | 2 | 3 | 4 | 5 | 6 | 7† | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | 28 | 76 | 41 | 40 | 43 | 52 | 43 | 33 | 43 | 37 | 42 |
| Gender | F | F | M | M | F | F | M | F | F | M | M |
| Overdose Type | S | A | S | U | A | A | S | S | S | A | S |
| APAP Dose, gm | - | - | 40 | - | - | 84 | 31.5 | 75 | 19.5 | - | - |
| APAP Level | 0 | - | 186 | - | 10 | 0 | - | 0 | 31 | 0 | 232 |
| Alcohol Use | N | N | Y | Y | Y | N | Y | Y | N | Y | - |
| Symptom to Coma Duration, days | 5 | 4 | 3 | 1 | 1 | 14 | 14 | 8 | 5 | 5 | 0 |
| Late Presenter | Y | N | N | N | N | Y | Y | Y | Y | Y | N |
| ALT, IU/L | 1154 | 576 | 13110 | 3779 | 9386 | 629 | 136 | 1810 | 621 | 4030 | 9810 |
| AST, IU/L | 485 | 1808 | 18710 | 10860 | 17780 | 862 | 129 | 170 | 277 | 3899 | 6910 |
| Bilirubin, mg/dL | 7.8 | 1.4 | 10 | 0.7 | 0.7 | 34.6 | 22.9 | 11.1 | 7.3 | 6 | 4.8 |
| APAP-adducts, nmol/mL | .207 | .632 | .246 | .342 | .294 | .157 | .010 | .335 | .446 | .019 | .244 |
| MELD score | 30.2 | 10.6 | 41.9 | 34.9 | 29.1 | 46.2 | 33.5 | 38.1 | 19.2 | 44.9 | 41.6 |
| APAP King’s Criteria met | N | N | N | N | N | N | N | N | N | N | Y |
| Hepatic Coma Grade | 4 | 2 | 1 | 4 | 3 | 3 | 1 | - | 3 | 3 | 4 |
| Ventilated | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Any NAC Treatment | Y | Y | Y | Y | Y | Y | N | N | Y | Y | Y |
| OLTx day # | 3 | 5 | |||||||||
| Death, day # | 5 | 28 | 9 | 5 |
If not listed as died or transplanted, the remaining patients survived without transplantation.
Dashes indicate information not available.
Subsequent assessment: probable autoimmune hepatitis leading to acute liver failure.
S= Suicidal, A=Accidental/Unintentional, U=Unknown
Acknowledgments
Financial Support: This study is supported by a cooperative research agreement from the National Institutes of Health: DK U-01-58369 and by the Northwestern Medical Foundation, Chicago, IL, and the Southwestern Medical Foundation, Dallas, TX.
Abbreviations
- ALF
acute liver failure
- APAP
acetaminophen
- APAP-CYS
acetaminophen-cysteine
- ALT
alanine aminotransferases
- MELD
model for end stage liver disease
- AST
aspartate aminotransferases
- NAC
N-acetylcysteine
Footnotes
Potential conflict of interest: Laura P James is developing a commercial assay for detecting acetaminophen adducts.
References
- 1.Trey C, Davidson CS. The management of fulminant hepatic failure. Prog Liver Dis. 1970;3:282–98. [PubMed] [Google Scholar]
- 2.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 3.Ostapowicz G, Fontana RJ, Schiødt FV, Larson AM, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 4.Stravitz RT, Kramer AH, Davern T, Shaikh AOS, Caldwell SH, Mehta RL, Blei AT, Fontana RJ, McGuire BM, Rossaro L, Smith AD, Lee WM. Intensive care of patients with acute liver failure: Recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007;35:2498–2508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 5.Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics. 1975;55:871–6. [PubMed] [Google Scholar]
- 6.Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–94. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30:446–51. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- 8.Pumford NR, Hinson JA, Potter DW, Rowland KL, Benson RW, Roberts DW. Immunochemical quantitation of 3-(cystein-S-yl) acetaminophen adducts in serum and liver proteins of acetaminophen-treated mice. J Pharmacol Exp Ther. 1989;248:190–6. [PubMed] [Google Scholar]
- 9.Pumford NR, Hinson JA, Benson RW, Roberts DW. Immunoblot analysis of protein containing 3-(cystein-S-yl) acetaminophen adducts in serum and subcellular liver fractions from acetaminophen- treated mice. Toxicol Appl Pharmacol. 1990;104:521–32. doi: 10.1016/0041-008x(90)90174-s. [DOI] [PubMed] [Google Scholar]
- 10.James LP, Alonso EM, Hynan LS, Hinson JA, Davern TJ, Lee WM, Squires RH. Detection of acetaminophen protein adducts in children with acute liver failure of indeterminate cause. Pediatrics. 2006;118:e676–81. doi: 10.1542/peds.2006-0069. [DOI] [PubMed] [Google Scholar]
- 11.James LP, Capparelli EV, Simpson PM, Letzig L, Roberts D, Hinson JA, Kearns GL, Blumer JL, Sullivan JE. Acetaminophen-associated hepatic injury: Evaluation of acetaminophen protein adducts in children and adolescents with acetaminophen overdose. Clin Pharmacol Ther. 2008;84:684–90. doi: 10.1038/clpt.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James LP, Letzig LG, Simpson PM, Capparelli E, Roberts DW, Hinson JA, Davern TJ, Lee WM. Pharmacokinetics of acetaminophen protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37:1779–84. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 14.O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–45. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 15.UNOS. [4-18-09];MELD/PELD Calculator Documentation (revised 1-28-09) http://www.unos.org/SharedContentDocuments/MELD_PELD_Calculator_Documentation.pdf.
- 16. [2-3-09];UNOS Policy 3.6 Organ Distribution: Allocation of Livers (revised 6-20-08) http://www.unos.org/policiesandbylaws/policies.asp?resources=true.
- 17.Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Davern TL, III, Murray NG, McCashland T, Reisch J, Robuck PR the ALFSG. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–864. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James LP, Simpson P, Russo M, Watkins PB. Detection of acetaminophen protein adducts in serum during therapeutic exposure to acetaminophen in healthy volunteers. Hepatology. 2007;46(suppl 1):812A. [Google Scholar]
- 19.Teo EK, Ostapowicz G, Hussain M, Lee WM, Fontana RJ, Lok ASF. Hepatitis B infection in patients with acute liver failure in the United States. Hepatology. 2001;33:972–76. doi: 10.1053/jhep.2001.23065. [DOI] [PubMed] [Google Scholar]
- 20.Umemura T, Tanaka E, Ostapowicz G, Brown KE, Heringlake S, Tassopoulos NC, Wang RY, Yeo AE, Shih JW, Orii K, Young NS, Hatzakis A, Manns MP, Lee WM, Kiyosawa K, Alter HJ. Investigation of SEN virus infection in patients with cryptogenic acute liver failure, hepatitis-associated aplastic anemia, or acute and chronic non-A-E hepatitis. J Infect Dis. 2003;188:1545–52. doi: 10.1086/379216. [DOI] [PubMed] [Google Scholar]
- 21.Lee WM, Brown KE, Young NS, Dawson GJ, Schlauder GG, Gutierrez RA, Fontana R, Rossaro L, Davern T, Lalani E. Brief report: No evidence for parvovirus B19 or hepatitis E virus as a cause of acute liver failure. Dig Dis Sci. 2006;51:1712–15. doi: 10.1007/s10620-005-9061-5. [DOI] [PubMed] [Google Scholar]
- 22.Levitsky J, Thadareddy A, Lakeman FD, Whitley RJ, Luby JP, Lee WM, Fontana RJ, Blei AT, Ison MG. Detection and diagnosis of herpes virus infection in adults with acute liver failure. Liver Transplant. 2008;14:1498–1504. doi: 10.1002/lt.21567. [DOI] [PMC free article] [PubMed] [Google Scholar]


