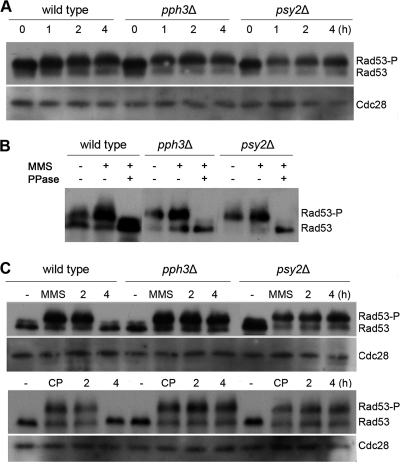
Fig. 6.
Pph3 and Psy2 regulate the phosphorylation state of Rad53 in C. albicans. (A) Wild-type (SJL9), pph3Δ (SJL7), and psy2Δ (SJL8) cells were incubated in YPD medium containing 0.02% MMS. Cells were harvested at the indicated time points for immunoblot analysis with anti-Myc antibody. Cdc28 was probed with anti-PSTAIRE antibody as a loading control. (B) Rad53 hyperphosphorylation in MMS-treated cells. The lysate of SJL9 (wild type with RAD53-Myc), SJL7 (pph3Δ RAD53-Myc), and SJL8 (psy3Δ RAD53-Myc) cells that had been grown in the presence of (+) 0.02% MMS for 4 h was divided into 2 parts. One was treated with λ-phosphatase (PPase), and the other was mock treated with the reaction buffer alone. The two samples, along with untreated cell lysates, were then subjected to Western blot analysis using anti-Myc antibody. (C) Wild-type (SJL9), pph3Δ (SJL7), and psy2Δ (SJL8) cells were incubated in YPD medium containing 0.02% MMS or 2 mM cisplatin for 6 h. Untreated cells (−) were included as a control. MMS and cisplatin were washed out, and the cells were resuspended in YPD medium at 30°C. The cells were harvested at the indicated time points for immunoblot analysis with anti-Myc antibody. Cdc28 was probed with anti-PSTAIRE antibody as a loading control.