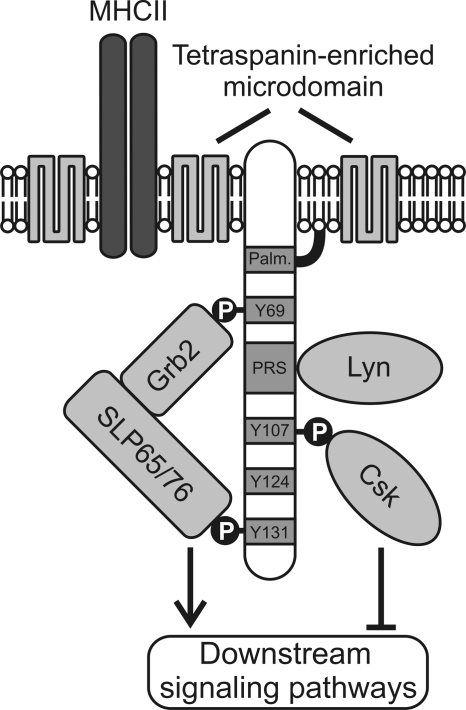
Fig. 8.
A model of SCIMP-mediated signal transduction. SCIMP is palmitoylated on its submembrane palmitoylation motif (Palm.) and is localized in tetraspanin-enriched microdomains together with MHC-II. Via its proline-rich sequence (PRS), SCIMP constitutively binds the SH3 domain of Lyn kinase. Upon cross-linking of SCIMP in the immunological synapse, SCIMP becomes phosphorylated (P) at several tyrosine residues and recruits a complex of Grb2 and SLP65 or SLP76 adaptors to the plasma membrane. Subsequently, SLP65 or SLP76 initiates a signaling cascade leading to activation of downstream signaling pathways. This process is controlled by the negative regulatory kinase Csk, which is also recruited to phosphorylated SCIMP.