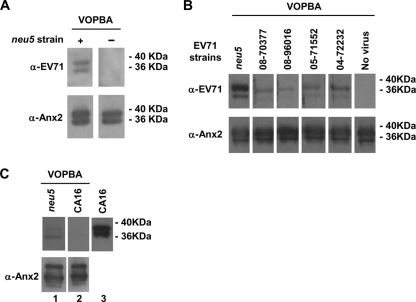
Fig. 2.
Interaction between clinical isolates and rAnx2 by VOPBA. (A) rAnx2 (0.5 μg per lane) was resolved on a nonreducing gel and was transferred to a nylon membrane for interaction with EV71 virions by VOPBA as described in Materials and Methods. (Top left) The bound EV71 was detected by an anti-EV71 (α-EV71) antibody. (Bottom) Subsequently, membranes were stripped with stripping buffer before immunoblotting using an anti-Anx2 antibody to confirm the colocalization of EV71 and Anx2 on the membrane. (Top right) A membrane that was not exposed to EV71 virions was used to validate the specificity of the anti-EV71 antibody. (B and C) Specific binding of rAnx2 to clinical isolates of EV71 (B) and lack of binding to CA16 (C). rAnx2 (0.5 μg in each lane) was resolved by 10% SDS-PAGE, transferred to a nylon membrane, and exposed to EV71 clinical isolates collected in 2004 (04-72232), 2005 (05-71552), and 2008 (08-70377 and 08-96016), along with neu5 as a positive control and CA16 to test for the specificity of binding. The virions bound on the membrane were detected by an anti-EV71 antibody or an anti-CA16 antibody (upper blots). As a positive control, the anti-CA16 polyclonal antibody was included in the Western blot analysis (C, blot 3; VP1 band, ∼38 kDa). The membrane was stripped with buffer and was reincubated with an anti-Anx2 antibody to validate the size of Anx2 (lower blots).