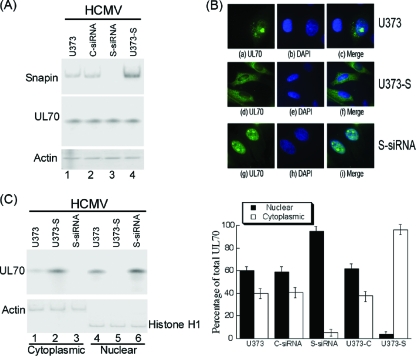
Fig. 6.
Effect of up- and downregulation of the expression of Snapin on the cellular distribution of UL70. (A) Western blot analysis of the levels of Snapin and Myc-tagged UL70 in the parental U373MG cells (U373) (lane 1), the Snapin-expressing U373-S cells (U373-S) (lane 4), or U373MG cells that were transfected with either anti-Snapin siRNA (S-siRNA) (lane 3) or control siRNA (C-siRNA) (lane 2). The expression of cellular actin was used as the internal control. Cells were transfected with pCMV-Myc-UL70 in the presence and absence of siRNAs. Forty-eight hours after transfection, cells were infected with HCMV at an MOI of 1. Protein samples were prepared at 48 to 72 h postinfection. (B) Immunofluorescence microscopy of the cellular localization of UL70 in the parental U373MG cells (U373), the anti-Snapin siRNA-treated cells (S-siRNA), or the Snapin-expressing cells (U373-S). Cells were infected with HCMV (MOI = 5) at 48 h posttransfection, fixed at 48 to 72 h postinfection, stained with antibodies, and visualized. The images of Myc-tagged UL70 (green) (a, d, and g) and the nuclei stained with DAPI (blue) (b, e, and h) were used to generate the composite images (c, f, and i). (C) Effect of Snapin on the distribution of UL70 in nuclear and cytoplasmic fractions. Different cells (e.g., parental U373MG cells, U373-S, and S-siRNA-treated cells) were transfected with pCMV-Myc-UL70 in the absence and presence of siRNAs. At 48 h posttransfection, cells were infected with HCMV (MOI = 1). At 48 to 72 h postinfection, cells were harvested and separated into nuclear and cytoplasmic fractions. Equivalent amounts of each fraction were analyzed by immunoblotting with anti-Myc. The purity of the nuclear and cytoplasmic fractions was assayed by immunoblotting with anti-histone H1 and anti-actin, respectively. The analyses were repeated three times. The standard deviation is indicated by the error bar (C, right panel).