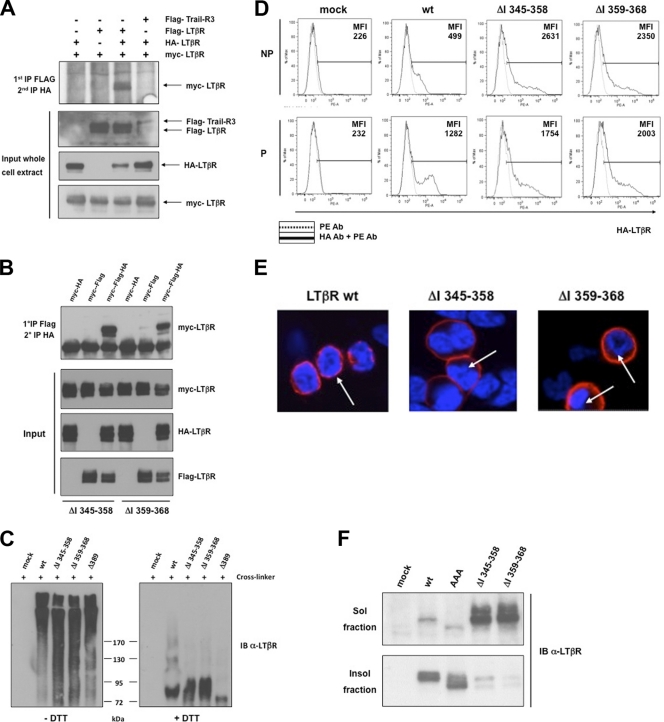
Fig. 3.
LTβR defective for p100 processing is sequestered into the plasma membrane. (A) HEK 293T cells were transfected with three differently tagged LTβRs (HA, Flag, and Myc tagged), and double immunoprecipitations were performed to analyze the trimerization of wt LTβR (see the supplemental material for details). (B) The same procedure as in panel A was applied for internal deletion mutants ΔI 345–358 and ΔI 359–368. (C) HEK 293T cells were transfected with wt LTβR, ΔI 345–358, ΔI 359–368, and Δ389. The cross-linker DSP was used prior to immunoprecipitation and immunoblotting of LTβR under nonreduced (−DTT) and reduced (+DTT) conditions. (D) Flow cytometry analysis of HEK 293 cells mock transfected or transfected with expression vector for wt LTβR, LTβR ΔI 345–358, or LTβR ΔI 359–368 and stained for cell surface LTβR (nonpermeabilized [NP]) or cell surface and intracellular LTβR (permeabilized [P]). MFI (mean fluorescence intensity) represents the value of one measurement out of three independent experiments. (E) Localization of wt LTβR, LTβR ΔI 345–358, and LTβR ΔI 359–368 in HEK 293 cells. Arrows indicate the perinuclear compartment. (F) Cell fractionation of LTβR into Triton X-100-soluble and -insoluble fractions from HEK 293 cells transfected with the indicated LTβR constructs.