Fig. 9.
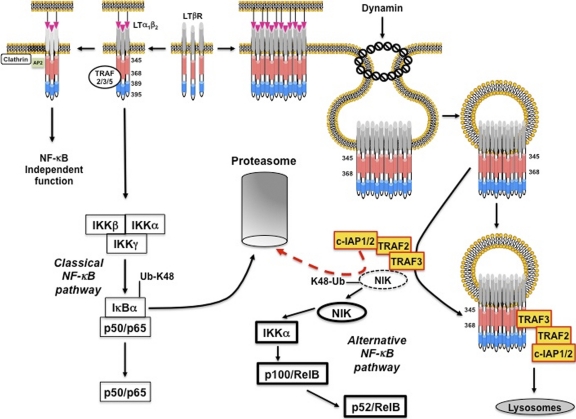
Model of LTβR trafficking and NF-κB activation. The binding of LTα1β2 to LTβR leads to its trimerization. This event allows a fast recruitment of TRAF proteins through the bipartite TRAF binding site (amino acids 389 to 395 in blue and 345 to 368 in red). This process then connects the receptor to the induction of IκBα degradation by the proteasome (classical NF-κB pathway). In the meantime, the complex AP2 in association with clathrin regulates an NF-κB-independent function of LTβR. While LTα1β2 accumulates at the cell surface of inducer cells, LTβR trimers form clusters on targeted cells. This process likely triggers the internalization of LTβR, which relies on the cytosolic region 345 to 368 and the presence of dynamin-2. Endocytic vesicles released from the plasma membrane expose the tail of LTβR toward the cytosol. This event allows LTβR to compete with intracellular NIK for the binding of its inhibitory complex TRAF3/TRAF2/c-IAP1/2. As a consequence, the constitutive proteasomal degradation of NIK (dashed line) is alleviated. Thus, NIK accumulates (solid line) and activates IKKα, and both events trigger the processing of p100 and the generation of p52/RelB. TRAF3/TRAF2/c-IAP1/2 complex is then degraded into lysosomes.