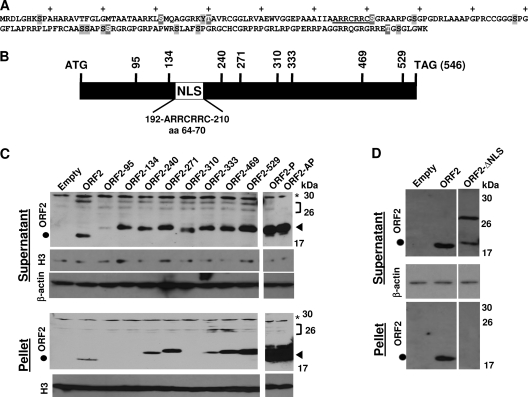
Fig. 1.
Generation of BHV-1 ORF2 mutants. (A) Amino acid sequence of ORF2. NLS (underlined), 15 putative phosphorylation sites (gray shaded amino acids), and 5 consensus protein kinase A (PKA) and/or PKC phosphorylation sites (gray shaded amino acids that have white lettering) are shown. The plus signs denote every 10th amino acid in ORF2. (B) ORF2 coding sequences (BamHI-SalI) were cloned into the pUC57 vector, and transposon linker insertion reactions were performed as described in Materials and Methods. Initial mapping was performed by restriction digestion, and the precise location of the transposon insertion was confirmed by sequencing. Vertical lines with numbers indicate nucleotide positions of transposon insertions. The relative position of the consensus nuclear localization signal (NLS) is denoted by the white rectangle. (C) The transposon mutants and the two phosphorylation mutants were cloned into the pCMV-Tag-2B vector and transfected into Neuro-2A cells. At 48 h after transfection, cells were collected and lysed using hypotonic buffer as described in Materials and Methods. After centrifugation, the supernatant was removed. The nuclei and other cellular debris were suspended in RIPA buffer, and the solubilized proteins were collected after centrifuging the residual debris; this solubilized fraction was designated the pellet. Detection of Flag-tagged ORF2 mutants was performed using an Anti-Flag antibody. A β-actin antibody was used to confirm that equal amounts of protein were loaded in each lane. A histone 3 antibody was used to identify nuclear proteins in the supernatant or pellet fraction. ORF2 is predicted to migrate as a 19-kDa protein, and the black circle denotes the position of this protein. The arrow denotes the higher-molecular-weight ORF2-specific bands that migrated at approximately 30 kDa, which were detected in certain samples. The transposon mutants are predicted to migrate as a 22-kDa protein and are denoted by a closed triangle. The location of a nonspecific band in D is denoted by the asterisk. The bracket denotes the position of the ORF2-specific bands that migrated slower than expected. For each lane, 100 μg protein was loaded. (D) Neuro-2A cells were transfected with the designated plasmids and the respective samples collected at 48 h after transfection as described above. These results are representative of more than 3 independent experiments.