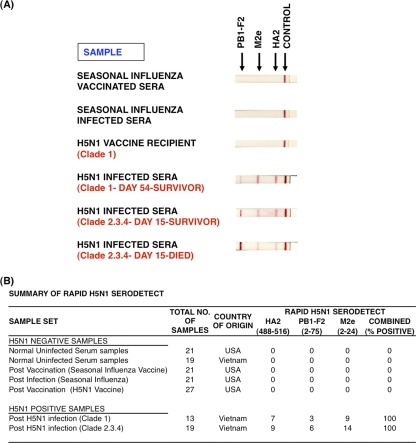
Fig. 6.
H5N1-SeroDetect rapid test for detection of H5N1 infections. (A) The rapid test was developed by striping H5N1 peptides [HA2 (488–516), PB1-F2 (2–75), and M2e (2–24)] and control protein (goat anti-human IgG), and data were assessed using a panel of H5N1 patient and control plasma samples at a 1:50 dilution. H5N1 virus-infected samples were from the survivors (clade 1 and clade 2.3.4) and individuals who died from infection (clade 2.3.4) in Vietnam. (B) Summary of reactivities of H5N1-negative and H5N1-positive samples in rapid assay format of H5N1-SeroDetect. Combined reactivity depicts positivity to any one or more antigens in the H5N1-SeroDetect. In all the H5N1 virus-infected individuals, the H5N1-SeroDetect rapid test was positive with at least one of the peptides.