Fig. 5.
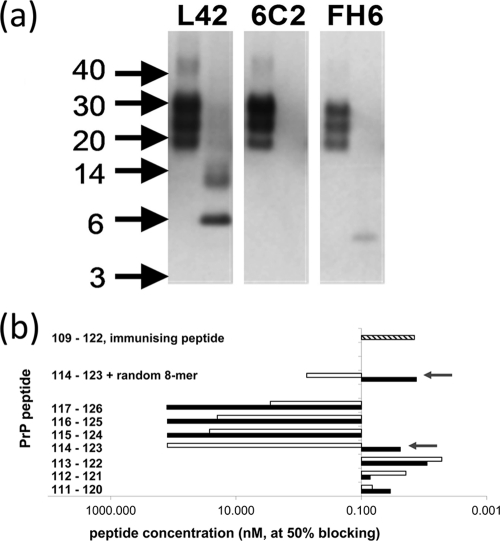
Evidence for cleavage at lysine residue 113 by Lys-C in PrPres of ovine scrapie brain material. (a) Electrophoretic pattern of PrP fragments after cleavage of ovine scrapie material with PK (left lanes) or PK followed by Lys-C (right lanes) on Western blots immunostained with antibodies L42, 6C2, and FH6. Surprisingly, the band reactive with L42 at 6 kDa was not reactive with 6C2 (Fig. 3). The absence of reactivity with C-terminal MAb FH6 at the 6-kDa position was as expected, and the reactive band below the 6-kDa position could well be an intermediate digestion product from codons 189 to 234 carrying a glycophosphatidyl anchor. In each lane, 0.5 mg TE was applied. Concentrations used for L42, 6C2, and FH6 antibodies were 0.1, 0.5, and 0.5 μg/ml, respectively. (b) Blocking ELISA to show the requirement of an N-terminal peptidyl link at lysine 114 of PrP for MAb 6C2 binding. Peptides with (shaded bars) and without (open bars) an amino-terminal N-acetyl group differed profoundly in their affinity only when residue 114 of ovine PrP is the N-terminal residue (arrows). A high blocking effect corresponds to peptide concentrations of about or below 0.1 μM at 50% blocking. Thus, the presence of the acetyl group appeared to be essential for 6C2 binding and was considered representative of an intact peptide bond. For comparison, other peptide sequences were tested, i.e., the peptide from codons 109 to 122 used for immunization as a positive control (hatched bar) and a peptide set containing the sequence from codons 114 to 123 at the C terminus, followed by an 8-amino-acid randomized sequence with or without an N-terminal N-acetyl group (the randomized sequence used as a control to exclude the influence of C-terminal variations in proximity to the epitope).