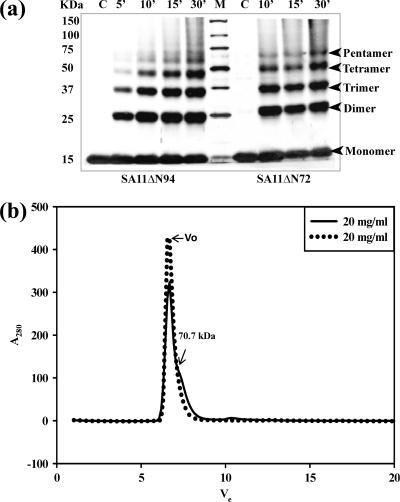
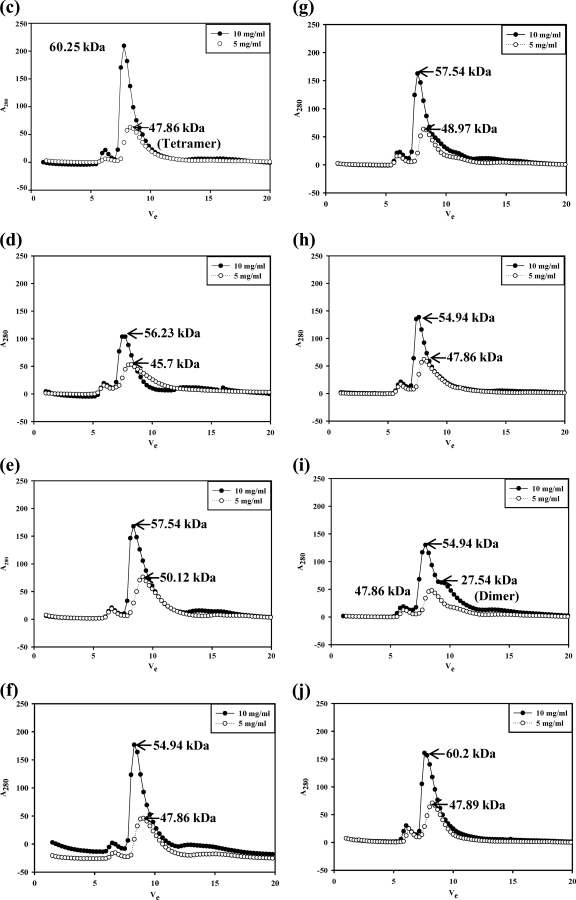
Fig. 9.
NSP4 exists in multiple oligomeric states, as demonstrated by glutaraldehyde cross-linking and analytical gel filtration of the SA11ΔN72 and SA11ΔN94 proteins by use of a Superdex G75 column. (a) Glutaraldehyde cross-linking. Bacterial cells expressing ΔN94 were lysed in a buffer containing 10 mM Tris-HCl (pH 7.5) and 100 mM NaCl in the absence of calcium, and bacterial cells expressing ΔN72 were lysed in the presence of 10 mM CaCl2, but both were purified further under identical conditions in the absence of added calcium. The proteins were cross-linked for the indicated time periods directly on the beads using an equimolar ratio of protein to cross-linker. The proteins were resolved by SDS-PAGE, and the bands were visualized by silver staining. Note the pentamers and other oligomeric forms of both mutant proteins purified after the lysis of cells in the presence or absence of calcium. M, Precision Plus protein standards from Bio-Rad. (b) Analytical size-exclusion chromatography of ΔN72. The protein was analyzed after purification from cells lysed in Tris-HCl (pH 7.5) buffer in the presence (−) or absence (.....) of 10 mM CaCl2. Vo, void volume. (c to j) Analytical size-exclusion chromatography of ΔN94 purified under different conditions. (c) Cells lysed in NaAc buffer (pH 5.6) (condition A); (d) lysis and purification in the absence of calcium (condition B); (e) lysis in the presence of 0.1 mM CaCl2 (condition C0.1); (f) lysis in the presence of 1.0 mM CaCl2 (condition C1); (g) lysis in the presence of 10 mM CaCl2 (condition C10); (h) lysis and purification in the presence of 0.1 mM CaCl2 (condition D); (i) lysis in the presence of 10 mM MgCl2 (condition E); (j) lysis and purification in the presence of 0.1 mM MgCl2 (condition F).