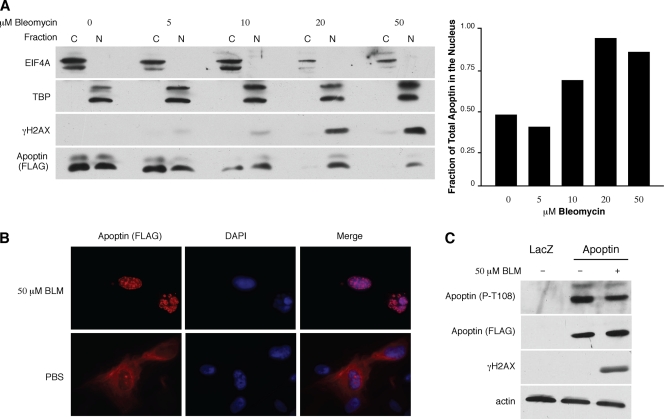
Fig. 2.
DNA damage induces nuclear localization of Apoptin in primary cells. (A) MRC5 fibroblasts were infected with Ad-Apwt and subsequently treated with the indicated concentrations of BLM for 18 h. The cells were then fractionated into cytoplasmic and nuclear fractions and separated by SDS-PAGE, and the levels of Apoptin present in each fraction were examined by immunoblotting with anti-FLAG antibody. EIF4A (eukaryotic translational initiation factor 4) and TATA box binding protein (TBP) are shown as markers for the cytoplasmic (C) and nuclear (N) fractions, respectively. Band intensities of Apoptin were quantitated from blots and graphed as the fraction of total apoptin present in the nucleus under each condition (right). (B) MRC5 fibroblasts were infected with Ad-Apwt and then treated with 50 μM bleomycin or PBS for 18 h. The cells were then fixed and stained with anti-FLAG antibody to detect Apoptin and DAPI to visualize nuclei. (C) MRC5 fibroblasts were infected and treated as described for panel A. Whole-cell lysates were separated by SDS-PAGE, and levels of phosphorylated Apoptin on threonine 108 (P-T108) were determined by immunoblot analysis using a phosphospecific antibody. Levels of total Apoptin were detected using anti-FLAG antibody; activation of DDR was confirmed with γH2AX, and an actin immunoblot was used as a loading control.