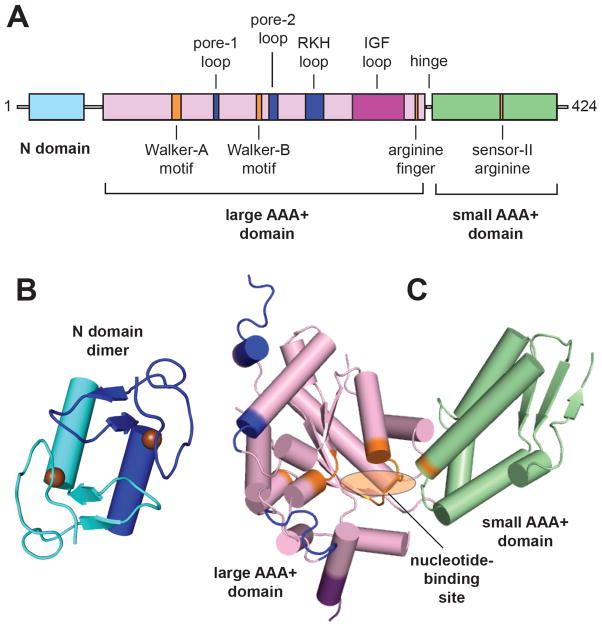
Fig. 2.
Domain structure of ClpX. (A) Arrangement of domains and characteristic functional motifs with respect to the linear sequence are shown for E. coli ClpX. Motifs are colored blue for ssrA-tag binding, orange for ATP binding and hydrolysis (orange), or purple for ClpP binding. The pore-2 loop is also involved in ClpP binding. (B) Structure of the N-domain dimer (1OVX) [16]. Spheres represent zinc atoms. (C) Structure of a AAA+ module in a single ClpX subunit (3HWS) [18]. Nucleotide binds in the cleft between the large and small AAA+ domains. Motif colors correspond to those in panel A.