Figure 4. Three major cell death morphotypes and their immunological profiles.
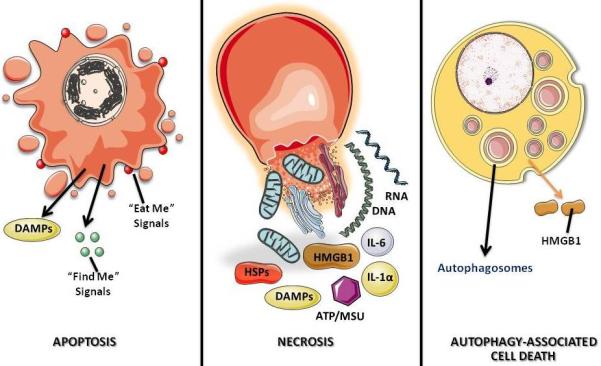
Apoptosis is morphologically characterized by chromatin condensation, cleavage of chromosomal DNA into internucleosomal fragments, cell shrinkage, membrane blebbing and formation of apoptotic bodies without plasma membrane breakdown. Typically apoptotic cells release “find me” and “eat me” signals required for the clearance of the remaining corpses by phagocytic cells. At the biochemical level, apoptosis entails the activation of caspases, a highly conserved family of cysteine-dependent aspartate-specific proteases. Necrosis is morphologically characterized by vacuolization of the cytoplasm, swelling and breakdown of the plasma membrane resulting in an inflammatory reaction due to release of cellular contents and pro-inflammatory molecules. Classically, necrosis is thought to be the result of pathological insults or be caused by a bio-energetic catastrophe, ATP depletion to a level incompatible with cell survival. The biochemistry of necrosis is characterized mostly in negative terms by the absence of caspase activation, cytochrome c release and DNA oligonucleosomal fragmentation. Autophagy is characterized by a massive vacuolization of the cytoplasm. Autophagic cytoplasmic degradation requires the formation of a double-membrane structure called the autophagosome, which sequesters cytoplasmic components as well as organelles and traffics them to the lysosomes. Autophagosome-lysosome fusion results in the degradation of the cytoplasmic components by the lysosomal hydrolazes. In adult organisms, autophagy functions as a self-digestion pathway promoting cell survival in an adverse environment and as a quality control mechanism by removing damaged organelles, toxic metabolites or intracellular pathogens.
