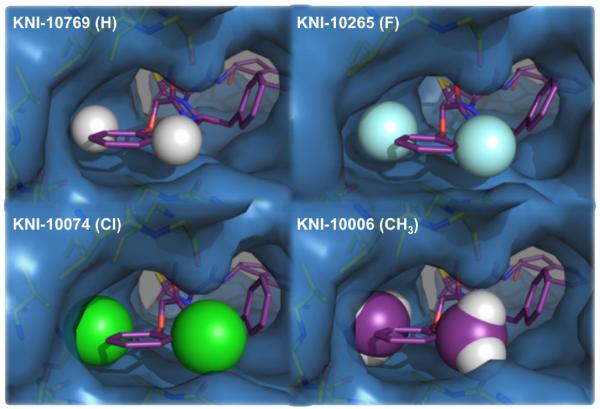
Figure 8.
Illustration of the progressive filling of the binding cavity by substituents with increasing van der Waals radii. The transition from hydrogen to fluorine is accompanied by an enthalpic gain and an entropic loss, reflecting the loss of conformational degrees of freedom once significant van der Waals contacts are established. The thermodynamic changes associated with fluorine, chlorine and methyl are monotonic and characterized by improvements in both the enthalpy and entropy changes. In all, the changes from hydrogen to methyl bring about a two order of magnitude in binding affinity. Increasing the substituent size beyond methyl (e.g. isopropyl) lowers the affinity by 5000-fold and transforms the binding enthalpy from favorable −6.5 kcal/mol to unfavorable +3 kcal/mol. In this figure, KNI-10769 (H) was modeled using the crystallographic structures of the other substituents.