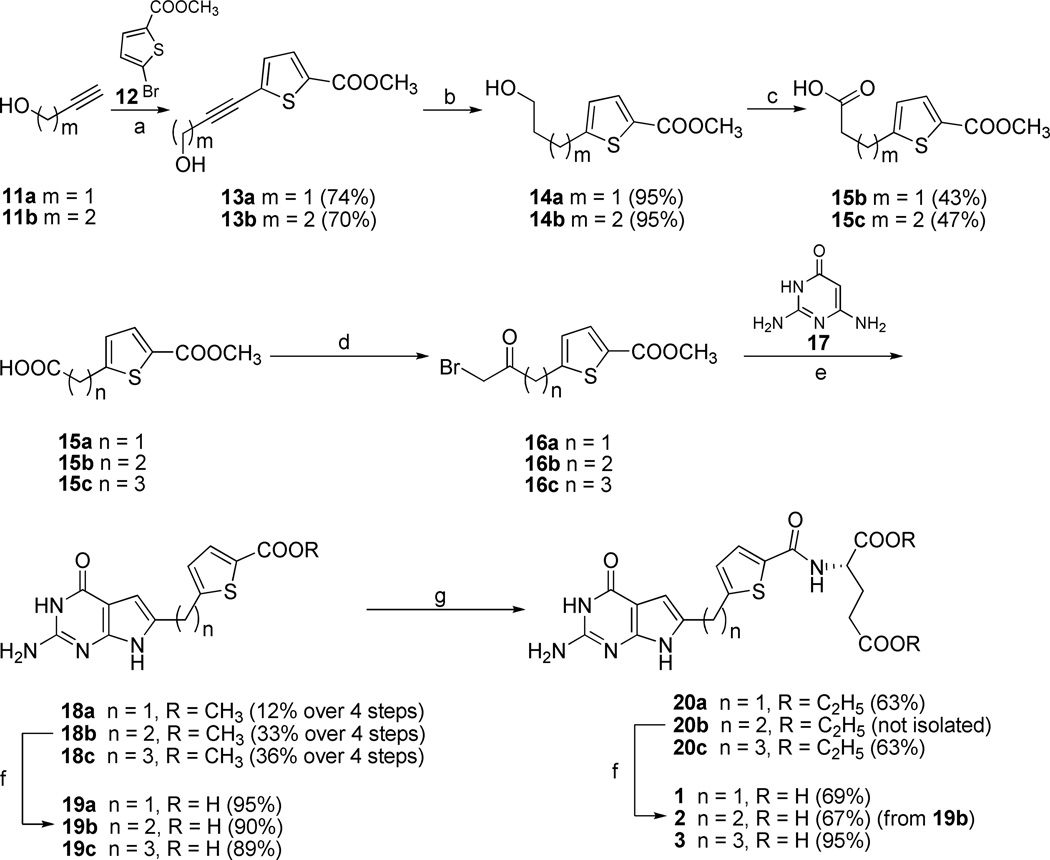
Scheme 1a.
a Reagents and conditions: (a) CuI, PdCl2, PPh3, Et3N, CH3CN, microwave,100 °C, 10 min; (b) 10% Pd/C, H2, 55psi, MeOH, 4 h; (c) H2SO4, CrO3, 0 °C~RT; (d) i. oxalyl chloride, CH2Cl2, reflux, 1 h; ii. diazomethane, Et2O, RT, 1h; iii. HBr, 70–80 °C, 2 h; (e) DMF, RT, 3 days; (f) i. 1N NaOH, RT, 12 h; ii. 1N HCl; (g) N-methylmorpholine, 2-chloro-4,6-dimethoxy-1,3,5-triazine, L-glutamate diethyl ester hydrochloride, DMF, RT, 12 h.