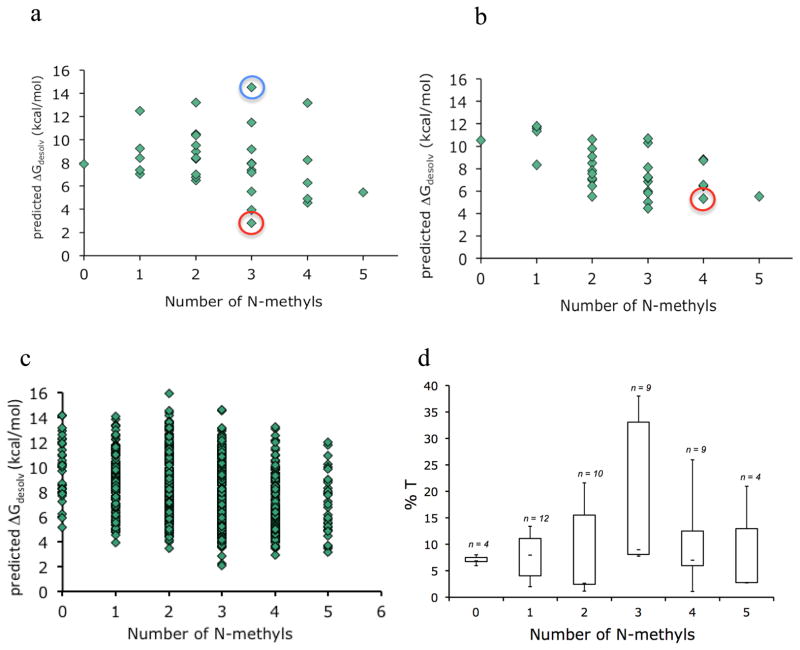
Figure 5.
Predicted free energies of desolvation of all N-methyl variants for scaffolds 1 (a) and 2 (b). Compounds 3 and 4 are indicated with red circles. Compound 7 is indicated with a blue circle. c) Predicted free energies of desolvation of all N-methyl variants for all diastereomers based on the sequence cyclo[D/L-Leu; D/L-Leu; D/L-Leu; D/L-Leu; D/L-Pro-LTyr]. d) Boxplot showing the PAMPA permeabilities (%T) as a function of the number of N-methyl groups for starting materials (0 N-methyls) and products (1–5 N-methyls) derived from the on-resin N-methylation of scaffolds 12, 19, 21, and 23 (Figure 2a) under three different solvent conditions: 1) LiOtBu (THF)/CH3I (THF), 2) LiOtBu (DCM)/CH3I (DCM), 3) LiOtBu (THF)/CH3I (DMSO).