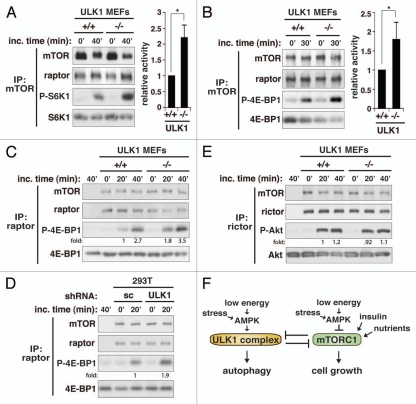
Figure 6.
ULK1 inhibits the kinase activity of mTORC1. (A) ULK1 has a negative effect on the kinase activity of mTORC1. mTOR was isolated from ULK1 MEFs. The kinase activity of mTOR was analyzed using S6K1 as substrate. The phosphorylation state of S6K1 at Thr389 was analyzed by protein gel blotting. The level of phosphorylation was quantitatively analyzed in the bar graph on the right-hand side. Values are means ± std from three independent experiments. *p < 0.05. (B) The inhibitory effect of ULK1 on mTORC1 kinase activity was assessed in vitro using 4E-BP1 as substrate. The bar graph represents quantitative assessment of the phosphorylation state of 4E-BP1 at Thr37/46. Values are mean ± std from two independent experiments. *p < 0.05. The mobility shift of raptor was unclear because we used a high perent of acrylamide in SDS-PAGE to analyze 4E-BP1. (C) Raptor immunoprecipitate isolated from ULK1 MEFs was analyzed for the kinase activity toward phosphorylation of 4E-BP1. (D) Raptor immunoprecipitate isolated from scrambled (sc) or ULK1 shRNA-transduced 293T cells was analyzed for the kinase activity toward phosphorylation of 4E-BP1. (E) Rictor immunoprecipitate isolated from ULK1 MEFs was analyzed for the kinase activity toward phosphorylation of Akt at Ser473. (F) Model for the reciprocal regulation between the ULK1 complex and mTORC1.