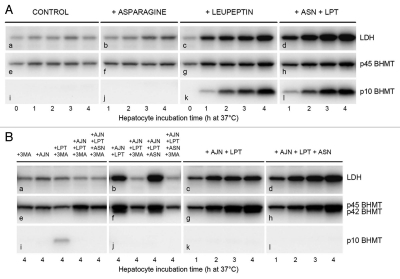
Figure 6.
Effects of AJN-230 and autophagic-lysosomal pathway inhibitors on autophagic sequestration and processing of BHMT and LDH in live cells. Hepatocytes were incubated at 37°C for up to 4 h (A and B) or for 2 × 90 min (C) in the presence of asparagine (ASN; 20 mM), leupeptin (LPT; 0.3 mM), 3-methyladenine (3MA; 10 mM), AJN-230 (AJN; 50 µM) or ammonium chloride (NH3; 20 mM) in various combinations. Cell corpse-associated LDH (top rows), BHMT forms (N-terminal antibody; second and third rows), legumain (fourth row) or cathepsin B (bottom row) were measured by immunoblotting. (A) Effect of asparagine on LDH accumulation and p10 fragment generation. Parts in the same row are from the same gel. (B) Effects of AJN-230 and asparagine on LDH accumulation and p42 accumulation. Parts b,f and j represent a different experiment than the other parts. (C) Inhibition of legumain activity by ammonia. Hepatocytes were first incubated for 90 min at 37°C with ammonium chloride, asparagine or leupeptin as indicated, then washed and reincubated for another 90 min at 37°C with ammonium chloride, leupeptin or 3MA as indicated. Samples from two different experiments (0–90 and 90–180, respectively) were applied to the same gel. (D) Schematic summary of autophagic BHMT and LDH processing. The results of the present study indicate that BHMT is processed in amphisomes: An initial modification (e.g., a C-terminal truncation of full-length p45 BHMT by an unknown, leupeptin-resistant proteinase produces a 42 kDa intermediate that is cleaved by legumain to generate an N-terminal 10-kDa and a C-terminal 32-kDa fragment, both being degraded by leupeptin-sensitive cysteine cathepsins. In addition, p45 and p42 can (sequentially or independently) be degraded by a legumain-independent, leupeptin-sensitive pathway. LDH is degraded in amphisomes by leupeptin-sensitive proteolysis and in lysosomes by leupeptin-resistant proteolysis. Both leupeptin and asparagine suppress amphisome-lysosome fusion, whereas vinblastine (VBL) inhibits the fusion of autophagosomes with later autophagic vacuoles. Ammonia (NH3) reversibly inhibits acid proteolysis in general by neutralizing amphisomes and lysosomes; in addition, the neutralization apparently causes irreversible inhibition of legumain.