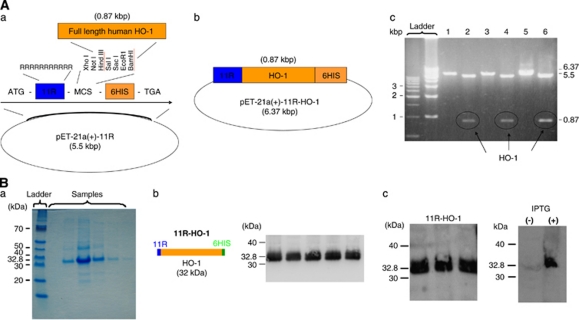
Figure 1.
(A) Structure of the 11R-HO-1 gene. (a) and (b) Structure of the pET21a(+)-HO-1-11R plasmid. (c) Results of electrophoresis. pET 21a(+)-HO-1-11R cut with Bam HI (1, 3, 5) and with Bam HI and Hind III (2, 4, 6). The cDNA encoding the therapeutic protein (human HO-1) is subcloned into pET 21a(+)-11R vectors digested two restriction enzymes. Plasmid pET-21a(+)-HO-1-11R was incubated at 37°C for 3 hours with Bam I and Hind III and then analyzed electrophoretically. (c) Bands of pET-21a(+)-11R-HO-1 (6.37 kbp), pET-21a(+)-11R (5.5 kbp), and human HO-1 plasmid (0.87 kbp). This result confirms human HO-1 cDNA subcloning into pET 21a(+)-HO-1-11R. ATG, start codon; 11R, 11 consecutive arginine residues (MRRRRRRRRRRR); MCS, multiple cloning site; 6HIS, 6 × histidine; TGA, stop codon. (B) 11R-HO-1 protein expression. Recombinant proteins were expressed in Escherichia coli BL-21 and purified using His Tag-Ni affinity under denaturing conditions. Proteins were dialyzed against PBS and used in biologic assays. Expression of the 11R-HO-1 protein was determined at 32.8 kDa by Coomassie brilliant blue staining of SDS-PAGE gels (a). Schema of the 11R-HO-1 protein (b, left). Protein was fused to 11R and a histidine tag for efficient protein purification. Western blotting proceeded using an anti-histidine antibody against the His tag part of the protein (b, right) and an anti-HO-1 antibody (c, left). After induction with 0.4 mmol/L IPTG, 11R-HO-1 was specifically expressed (c, right). 11R, 11 consecutive arginine residues; HO-1, heme-oxygenase-1; IPTG, isopropyl-1-thio-β--galactopyranoside; PBS, phosphate-buffered saline.