Abstract
Higher rates of low birth weight and prematurity are observed in pregnancies generated with assisted reproduction technologies (ART). Both conditions have been associated with placental inflammation and oxidative stress. Since placental and fetal levels of progesterone, a major anti-inflammatory steroid, are decreased in murine ART, we investigated placental inflammation and oxidative stress in this model as potential mediators of negative birth outcomes. After generating mouse pregnancies by in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) we evaluated the antioxidant defense network and major inflammatory cytokines in maternal, placental and fetal tissues. Additionally, placentas were analyzed for total lipid levels, fibrosis, apoptosis, reactive oxygen species and integrity of intracellular nucleotides. Placentas from ART contained significantly less lipids, with greater levels of apoptosis and degraded nucleotides. Placentas from ICSI pregnancies had lower activities of superoxide dismutase (SOD), thioredoxin reductase (TrxR), xanthine oxidase (XO), catalase, glutathione-S-transferase (GST) glutathione peroxidase, and glutathione reductase (GR). Furthermore, GR, GST and SOD were also lower in fetal livers from ICSI pregnancies. Placentas from IVF pregnancies had decreased levels of SOD, TrxR and XO only. In placentas from both ICSI and IVF pregnancies IL-6 levels were significantly increased. These data suggest that ART is associated with placental inflammation (IL-6), oxidative stress and apoptosis but not fibrosis or remodeling. These effects are markedly greater with the ICSI technique. Since ICSI is ubiquitous, oxidative stress and placental inflammation associated with this method may be a critical factor in negative birth outcomes such as prematurity and low birth weight.
Keywords: Apoptosis, ICSI, IL-6, inflammation, IVF, placenta, oxidative stress
Introduction*
Despite being viewed as a relatively safe and effective way to conceive for infertility, pregnancies achieved by in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) are associated with higher incidence of induced labor, Caesarean section, premature birth, small-for-gestational age babies, pediatric cancer, imprinting disorders and congenital abnormalities [1–4].
Although the mechanism for higher rates of negative outcomes occurring in pregnancies where assisted reproduction technologies (ART) are used is unknown, several studies implicate placental dysfunction as a critical factor. Higher placental weight, delayed placental development (without structural abnormalities), decreased steroid levels in the fetal compartment, increased placental steroid glucuronidation and altered placental metabolism have been documented in mice [5–9]. In humans, higher rates of placenta previa, abruption, premature rupture of the membranes, preeclampsia, unusual shape and umbilical cord insertion have also been reported [3, 10, 11]. Therefore, elucidating the effects of assisted reproduction on placental function is a promising avenue for unraveling the mechanisms behind unsatisfactory pregnancy outcomes associated with ART.
In terms of placental research, mouse and human structures are similar in form and function, but structurally different. According to Grosser's classification, murine placentas are hemotrichorial. The maternal decidua and spongiotrophoblast are partially embedded in the endometrium and maternal-fetal exchange occurring in the labyrinth, consisting of syncitiotrophoblasts, chorionic trophoblasts, stroma, and blood vessels [12]. In humans, the classification is hemomonochorial since the placenta is not embedded and the chorionic villi are bathed directly in maternal blood [12]. In spite of these differences placental function is similar and it remains vital for fetal well-being in both species, due to its critical role in transport of substances (including steroids and other hormones, nutrients and essential minerals) to the fetus, as well as removing wastes.
A previous key finding in this same cohort of ART animals was that progesterone levels were decreased in the placenta, but not systemically in the mother or fetus [8]. In pregnancy, progesterone suppresses inflammatory responses [13, 14] and prevents premature parturition and fetal growth restriction [15–19]. We hypothesized that negative outcomes from ART may be mediated by placental inflammation and oxidative stress. Herein, using a mouse model of ART, we demonstrate that placental antioxidant defense enzymes are severely compromised with concurrently greater placental inflammation, lower placental lipid, increased apoptosis and degraded intracellular nucleotides. These data confirm inflammation and oxidative stress as contributors to placental dysfunction in ART, which may provide a mechanism for the higher incidence of negative reproductive outcomes.
Materials and Methods
Reagents
Mineral oil was purchased from Squibb and Sons (Princeton, NJ); pregnant mares' serum gonadotrophin (eCG) and human chorionic gonadotrophin (hCG) were from Calbiochem (Spring Valley, CA); assay kits for antioxidant enzymes were from Cayman Chemical Company (Ann Arbor, MI). All other chemicals were obtained from Sigma Chemical Co. (St Louis, MO) unless otherwise stated.
Animals, in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI)
The animals used and IVF/ICSI methods were previously described. However, the current results are novel and do not in any way duplicate the work reported in the previous paper [8]. Briefly, B6D2F1 mice (C57BL/6 × DBA/2) and CD-1 mice were obtained at 6 weeks of age from National Cancer Institute (Raleigh, NC) and Charles River Laboratories (Wilmington, MA), respectively. Sperm and oocytes came from B6D2F1 donors, and CD-1 females were surrogate mothers. Animals were fed ad libitum with a standard diet and maintained in a temperature and light-controlled room (22°C, 14 h light/10 h dark). These studies were approved by the Institutional Animal Care and Use Committee at the University of Hawaii.
Tissue collection and processing
Tissues were collected at term (gestational day 18.5) by caesarian section. Tissues were washed in Dulbecco's PBS (D-PBS), drained and placed into tubes. During collection all samples were kept on ice up to 30 min before freezing at −80 °C. For sectioning, placentas were embedded in OCT medium, snap-frozen with Cyto-freeze (EMS, Hatfield, PA) and sectioned to 7 μm thickness at the John A. Burns School of Medicine Histology Core.
Tissue lysates were prepared by thawing and homogenizing tissues 1:4 (w:v) in Tris-HCl, 5 mM MgCl2, 2 mM PMSF buffer (pH 7.4). Aliquots were stored frozen at −80 °C until use. All lysates were normalized to 2 mg/mL protein using the bicinchoninic acid method with bovine serum albumin as the protein standard [20].
Histological examination of placental lipids and apoptosis
Lipid accumulation was visualized with Nile red [21]. Briefly, Nile red solution was prepared in acetone then diluted to 1 μg/mL working solution in 0.1M Tris (pH 7.4). A100 μL aliquot of working solution was pipetted onto each section, incubated for 10 min, washed (2×5 min, PBS) and fluorescence observed with a Vanguard 1400FL microscope (Mel Sobel Microscopes, NY). Images were captured with constant fluorescence excitation and exposure time using a Sony DSCP8 digital camera fitted to the trinocular lense. Analysis was performed on duplicate slides, three fields per slide from n = 4 (normal), and n = 6 (IVF and ICSI) placentas. Fluorescence intensity:tissue area ratios were generated using Image J (http://rsb.info.nih.gov/ij/) on original .jpg files with geometric means for each placenta used for graphing. Fluorescent images presented are original .jpg files except that images were uniformly rotated perpendicular to the visual plane with the chorionic plate to the right.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was performed with an in situ detection kit as per the manufacturer's instructions (BD Pharmigen, San Jose, CA). Duplicate slides from normal fertilization, IVF and ICSI (as above) were visualized using bright field microscopy (Olympus BX40, Melville, NY) fitted with a Sony DSCP10 camera and uniform light levels/exposure time. Stained dUTP nicks:tissue area pixel ratios were generated with Image J (http://rsb.info.nih.gov/ij/) using original picture files. For pictures presented, contrast and brightness were increased across the entire panel for printed visual impact.
ELISA detection of inflammatory cytokines
The inflammatory cytokines TNFα, IL-1β, IL-6 and GM-CSF were detected with commercial ELISA (BioLegend, San Diego, CA) on a subset of tissues comprising of maternal livers (n = 3 each for normal, IVF and ICSI), placentas (n = 6, 9 and 9 for normal, IVF and ICSI respectively), and fetal livers (n = 9 each for normal fertilization, IVF and ICSI respectively). For placentas and fetal livers, equal samples were taken from each of the three dams. ELISAs were accepted if the standard curve fit the 4-parameter logistic regression acceptably (R2 ≥ 0.98) and duplicate samples vary ≤ 15%.
Reactive oxygen species (ROS) levels
The dichlorofluorescein diacetate method for determining ROS was performed at 485/535 nm excitation/emission, every 20 sec for 10 min [22]. Results were transformed by comparison to standard curves of dichlorofluorescein (1 – 5000 nM; r2 = 0.988 ± 0.008, n = 4).
RNA integrity
RNA was extracted from 15 mg of tissue for all placentas. Extractions proceeded over multiple days using MoBio RNA Isolation Kits (MoBio, Carlsbad, CA, same lot number). On each day placental RNA from normal fertilization, IVF and ICSI pregnancies was extracted in parallel using the same kit, then concentrations and purity assessed by Nanodrop (Thermo Scientific, Wilmington, DE). All placentas were collected on the same day under the same conditions, stored identically and processed in parallel from the same kits.
Biochemical assays for antioxidant enzymes
Seven enzymes, Catalase (CAT), Glutathione Peroxidase (GPx), Gluthathione Reductase (GR), Glutathione-S-Transferase (GST), Superoxide Dismutase (SOD), Thiorodoxin Reductase (TrxR), and Xanthine Oxidase (XO) were tested in maternal livers (n = 3 each for IVF, ICSI and normal fertilization), placentas and fetal livers from IVF (n = 31), ICSI (n = 21) and normal fertilization (n = 20) using commercial kits as per the manufacturer's instructions (Cayman Chemical Company, Ann Arbor, MI).
These assays were validated using positive controls on each plate, and the variability of the assay determined using coefficient of variation (CV) – [(standard deviation/mean)*100]. The variability of assays was as follows: CAT intra-day CV 0.1%, n = 4, inter-day CV: not determined, all samples assessed on same day; GPx intra-day CV 19%, n = 4 and inter-day CV 21.5%, n = 3; GR intra-day CV 14.9 %, n = 4, inter-day CV 14.5%, n = 3; TrxR intra-day CV 23.7 % n = 4, inter-day CV 30.8%, n = 3. The enzymes SOD and XO were performed using commercial kits that do not include positive controls, for these assays results were accepted when CV of the triplicates was ≤15%.
Total GST activity was assessed with the substrate 1-chloro-2,4-dinitrobenzene with ε = 9.6 mM/cm [23, 24]. The positive control was human liver cytosol. Intra-day CV = 2.4 % (n = 3) and inter-day = 2.7% (n = 2).
Statistical analyses
Statistical analyses were performed using Prism 5.0 with α = 0.05. (GraphPad Prism, San Diego CA). Two-tailed student-t tests were used since all data approximated Gaussian distributions (D'Agostino-Pearson).
Results
Production of fetuses after mating, IVF and ICSI
As previously described [8], three females were mated with all becoming pregnant, providing 26 fetuses. When embryos produced by IVF and ICSI were transferred into the oviducts of pseudopregnant females, all females (3 per group) became pregnant with 37 (IVF) and 25 (ICSI) fetuses produced. Placenta weights averaged 89.5 ± 12.5 mg in normally conceived fetuses (n = 26) and were significantly higher in IVF (104.9 ± 19.7, P < 0.001, n = 37) and ICSI (108.6 ± 19.1 mg, P < 0.001, n = 25). There were no differences in placental weight between IVF and ICSI (P = 0.46). In contrast, fetal liver weights, which were used as a proxy for fetal weight, averaged 57.0 ± 13.6 g in normal fetuses and were not different in IVF fetuses (56.3 ± 10.8 g). However in ICSI fetal livers were significantly larger (75.6 ± 14.2 g, P < 0.01 vs. both normal and IVF). There were no significant differences in the number of fetuses, abortion sites or total embryos implanted between IVF and ICSI. However, IVF and ICSI pregnancies had more abortion sites than naturally mated females (8 and 11 vs. 1, respectively). Since the number of fetuses per dam did not differ between or among any group the effects observed in this study cannot be attributed to overcrowding.
Histochemistry
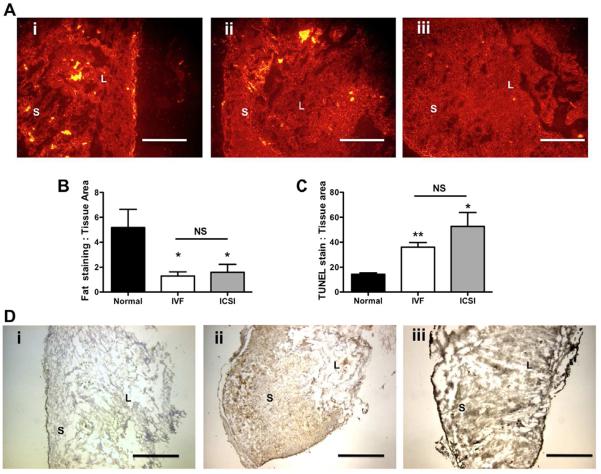
Placentas from ART had significantly lower total fat compared to normal fertilization (ICSI: P = 0.033, IVF: P = 0.013, Figure 1Ai – iii). Fat levels were not region-specific and differences in lipid accumulation between fertilization types were not observed. Image analysis of Nile red staining showed significantly less lipids in ART placentas as compared to normal reproduction (Figure 1B).
Figure 1. Immunofluorescence and histopathology analyses of placental lipid loading and apoptosis in placentas from normal and assisted fertilization pregnancies.
1A: Representative immunofluorescent images of mouse placentas stained with Nile Red at 10× magnification oriented with the chorionic plate to the right in all slides (scale bar = 250 μm) (i) Normal fertilization, (ii) IVF, (iii) ICSI. 1B: Semi-quantification of fluorescence using fluorescence:tissue area pixel ratio. Bars are means ± SEM. 1C: Semi-quantification of apoptosis measured by TUNEL staining:tissue area pixel ratio analyzed in Image J. 1D: Representative micrographs of TUNEL staining in the mouse placenta at 4× magnification (i) Normal fertilization, (ii) IVF, (iii) ICSI. Scale bar = 1 mm. Bars are means ± SEM. * = P < 0.05, ** = P < 0.01 vs. normal fertilization, t-test. Abbreviations S = syncytium, L = labyrinth zone. NS = not significant.
With light microscopy we did not observe overt histological changes in the placentas such as changes in fibrin deposits, syncytial knots or overt endothelial changes. Using TUNEL staining with DAB detection, higher levels of apoptosis were observed in placentas from ART pregnancies (P = 0.002 IVF and P = 0.024 ICSI, Figure 1C) compared to placentas from normal fertilization, which can be observed visually (Figure 1Di–iii).
Oxidative stress and antioxidant enzymes
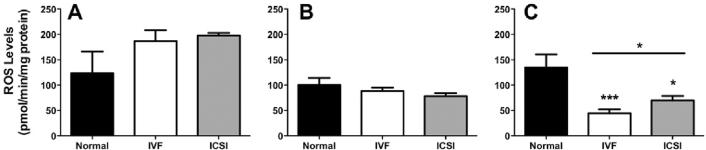
No significant differences in reactive oxygen species (ROS) between normal fertilization and ART were observed in placentas. However, maternal livers from normal fertilization had less ROS than maternal livers from ART, with the difference reaching statistical significance in ICSI (P = 0.04) and approaching in IVF (P = 0.08).
In fetal livers from ART pregnancies, total ROS levels were reduced 77% in IVF (P = 0.0002) and 48% in ICSI (P = 0.02), with IVF also being significantly lower than ICSI (P = 0.04, Figure 2).
Figure 2. Total reactive species in maternal-placental-fetal tissues from normal and assisted fertilization pregnancies.
A: Maternal liver; B: Placenta; C: Fetal liver. Bars are means ± SEM of n = 20, 31 and 21 for normal fertilization, IVF and ICSI placentas and fetal livers, and n = 3 for maternal livers. * = P < 0.05, ** = P < 0.01, *** = P < 0.001 vs. normal, t-test.
Table 1 shows the levels of antioxidant enzyme activities normalized per milligram of protein for seven enzymes, representing the entire network of antioxidant defense enzymes (except for nitric oxide synthase). Enzyme activities were generally highest in maternal livers, then comparable in placentas and fetal livers with the exception of xanthine oxidase where placental activities were greatest.
Table 1.
Activities of antioxidant enzymes in the maternal-placental-fetal unit in ART.
| TISSUE | CAT μmol | GPx nmol | GR nmol | GST nmol | SOD U | TrxR nmol | XO fmol | |
|---|---|---|---|---|---|---|---|---|
| Maternal Liver | Normal | 6.6 ± 3.5 | 325 ± 65 | 2.1 ± 0.3 | 117 ± 16 | 3.1 ± 1.4 | 4.7 ± 2.1 | 22 ± 1.2 |
| IVF | 5.1 ± 0.6 | 385 ± 96 | 1.6 ± 0.3 | 198 ± 29 * | 2.9 ± 1.0 | 4.7 ± 2.4 | 21 ± 2.6 | |
| ICSI | 9.1 ± 2.9 | 482 ± 108 | 2.2 ± 0.2 | 92 ± 14 | 3.4 ± 1.0 | 8.8 ± 4.3 | 17 ± 1.1 | |
| Placenta | Normal | 1.2 ± 0.1 | 251 ± 10 | 1.1 ± 0.1 | 19 ± 2.8 | 3.2 ± 0.6 | 17 ± 0.6 | 50 ± 4.0 |
| IVF | 1.4 ± 0.1 | 255 ± 9 | 0.9 ± 0.08 | 17 ± 1.5 | 1.5 ± 0.1 *** | 11 ± 0.4 *** | 23 ± 1.3 *** | |
| ICSI | 0.9 ± 0.03 * | 203 ± 5 *** | 0.6 ± 0.06 ** | 11 ± 0.8 * | 1.7 ± 0.2 * | 12 ± 0.9 *** | 27 ±2.4 *** | |
| Fetal Liver | Normal | 1.2 ± 0.2 | 280 ± 25 | 1.6 ± 0.25 | 39 ± 4.6 | 1.0 ± 0.2 | 4.6 ± 0.5 | 3.3 ± 0.4 |
| IVF | 1.7 ± 0.2 | 245 ± 20 | 0.9 ± 0.13 * | 31 ± 2.6 | 1.0 ± 0.1 | 5.2 ± 0.5 | 3.4 ± 0.4 | |
| ICSI | 1.4 ± 0.1 | 259 ± 21 | 0.7 ± .12 ** | 29 ± 4.1 * | 0.6 ± 0.1 * | 6.2 ± 0.9 | 3.0 ± 0.3 |
aAll enzyme activities are normalized to min−1mg protein−1 and expressed as mean ± SEM, with n= 3 for materna livers from each group and n = 20, n = 31 and n = 21 matched samples for placentas and fetal livers from normal fertilization, IVF and ICSI pregnancies, respectively. Abbreviations: μmol; micromoles, nmol; nanomoles, fmol; femtomoles, U = Units (amount of enzyme required to inhibit 50% of dismutation of superoxide). Statistical significance:
P < 0.05
P < 0.01
P < 0.001 vs. normal tissues, and significant changes are bolded.
Overall, consistent and significant effects of ART were noted whereby placental antioxidant enzyme activities were moderately decreased in IVF and severely compromised in ICSI. Additionally, a moderate reduction of fetal systemic antioxidant enzyme capacity in ICSI was indicated but no consistent changes to antioxidant enzyme capacity were observed in maternal livers.
RNA concentrations and integrity
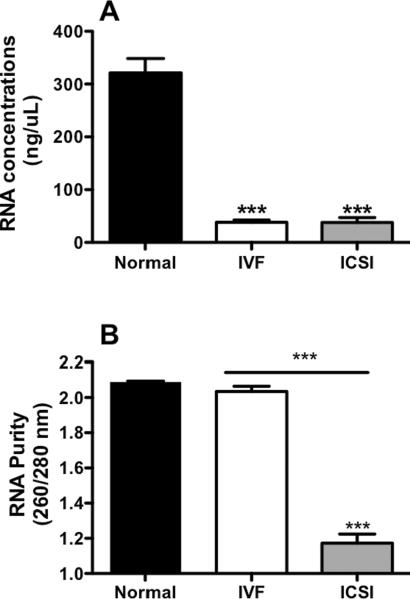
Total RNA extracted from placentas generated by ART was approximately 10-fold lower in concentration than RNA from placentas from normal fertilization pregnancies (both IVF and ICSI, P < 0.0001, Figure 3A). Furthermore, using the 260/280 ratio to assess the purity of RNA extracted, RNA purity was acceptably high in placentas from normal fertilization and IVF but around 45% lower in placentas from ICSI (P < 0.0001 vs. normal and IVF, t-test, Figure 3B).
Figure 3. Levels and purity of RNA extracted from 15 mg tissue samples of placenta from normal and assisted fertilization pregnancies.
3A: Concentration of RNA extracted from placentas after one round of extraction and clean-up. 3B: Integrity and purity of RNA (measures using 260/280 ratio). Bars are means ± SEM of n = 20, 31 and 21 for placentas from normal fertilization, IVF and ICSI. *** = P < 0.001, t-test.
Pro-inflammatory cytokines
The levels of four major pro-inflammatory cytokines (IL-6, TNFα, GM-CSF and IL-1β) were measured in maternal livers, placentas and fetal livers (Figure 4A–D). The trend for levels of all cytokines was as follows: highest levels in maternal livers, followed by fetal livers, and then placentas.
Figure 4. ART-mediated changes in pro-inflammatory cytokines in maternal livers, placentas and fetal livers.
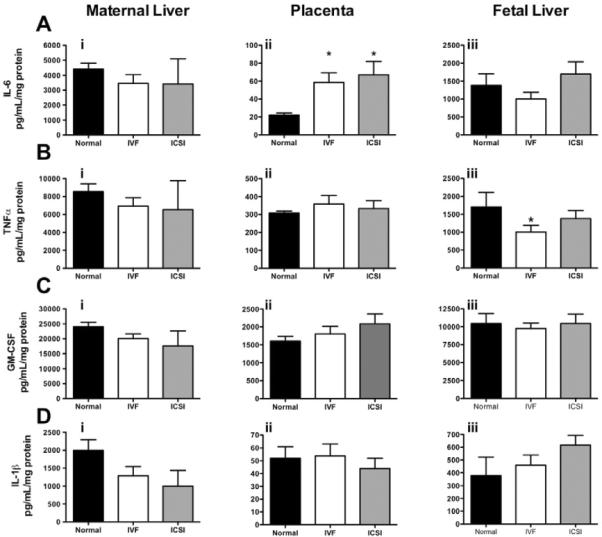
4Ai–iii: TNFα; 4Bi–iii: IL-6; 4Ci–iii GM-CSF; 4Di–iii IL-1β. Bars are means ± SEM of n = 9 (3 each from 3 separate litters) for fetal livers and placentas, except for placentas from normal fertilization, which are n = 6 (2 each from 3 separate litters) and maternal livers (n = 3). * = P < 0.05 vs. normal, t-test.
IL-6 levels were increased 3-fold in placentas from both IVF (P = 0.01) and ICSI (P = 0.02) pregnancies compared to normal fertilization, with no changes in maternal and fetal livers (Figure 4Ai–iii). Levels of TNFα were 40% lower in fetal livers (P = 0.04) from IVF and 20% lower from ICSI pregnancies compared to normal fertilization (Figure 4Biii) with no changes placentas or maternal liver. Although no significant differences were observed for GM-CSF and IL-1β, a trend towards increasing GM-CSF concentrations in placentas from ART occurred (Figure 4Cii). Likewise decreasing IL-1β in maternal livers (Figure 4Di) with increasing IL-1β levels in fetal livers (Figure 4Diii) was apparent in ART as compared to normal fertilization, with ICSI consistently reporting higher effects than IVF tissues.
Discussion
This study suggests that placental inflammation and oxidative stress are significantly raised in the mouse model of ART. Although no structural abnormalities in placentas from ART pregnancies have been noted here, or by others [5, 8], further testing revealed significantly lower placental lipid loading, increased placental cell death (apoptosis) and compromised intracellular nucleotides (lower RNA levels and integrity, higher DNA damage). Furthermore, ART manipulation resulted in increasing inflammation through the IL-6 pathway and greater oxidative stress in placentas, both of which were particularly apparent in ICSI placentas.
We have previously demonstrated that in murine pregnancies produced through ART, the placenta has greater capacity to metabolize and remove steroids through glucuronidation [7] and that this phenomenon is associated with lower steroid hormone levels transiting the placenta to the fetal unit [8]. These findings led to our current study since steroid hormones, especially progesterone, prevent oxidative stress and inflammation in pregnancy [13, 14, 25].
The importance of progesterone in reproductive biology was first established by Gregory Pincus in the 1950's [26]. The role of progesterone was further established when pregnant mice were subjected to ovariectomy and maintained with varying levels of exogenous progesterone and estrogen [17]. When progesterone levels were insufficient, placental inflammation and oxidative stress occurred, ultimately leading to miscarriage or pre-term birth [17]. These previous studies along with work from our own laboratory [7, 8] support for our current findings suggesting that placental inflammation, oxidative stress and dysfunction takes place secondary to ART-mediated reduction in placental progesterone and cholesterol.
Despite acknowledged structural differences between murine and human placentas, metabolism and transport functions of the organ are similar between the two species. It has recently been demonstrated that placental size is different in murine ART, but placental structures are not [5, 8]. Also, similar data have been demonstrated for human placentas where light microscopy also showed no major structural changes. However, when transmission electron microscopy was used the authors reported degeneration of syncytiotrophoblast terminal villi causing a thicker placental barrier, decreased apical microvilli and increased multiple vacuoles [27]. Our initial speculation was that greater placental weights were due to lipid accumulation. This is empirically sensible in an oxidative stress paradigm because accrual of intracellular fat is a known cause of oxidative stress, lipid peroxidation and tissue damage [28]. Somewhat surprisingly, our histological and biochemical analyses of placentas demonstrated significantly lower lipid: tissue ratio, and lower total lipids in ART. Hence, higher placental weights and oxidative stress in ART are not caused by increased intracellular lipids.
On the other hand, emerging research suggests that deficiencies in essential fats and cholesterol can also cause oxidative stress [29], in part because molecules such as high-density lipoprotein 3 (HDL3) which prevents lipid peroxidation and protein adduction, are unavailable when tissues are fat-depleted [29]. Our data seem to support this latter model, and also support the previous study demonstrating that cholesterol is depleted in murine ART placentas [8]. In the mouse, placental and fetal cholesterol is derived primarily from the maternal circulation with very low levels of fetal production. Furthermore, murine fetuses depend on transfer of maternal cholesterol throughout gestation with lack of maternal cholesterol transport leading to fetal death, even in late gestation [30#x2013;32]. In translating these data, humans are less dependent on maternal cholesterol in the third trimester, although fetal abnormalities can occur if cholesterol transport is insufficient [33]. Consequently, we speculate that the placental inflammation and oxidative stress in ART occurs through decreased cholesterol and essential lipid/lipoprotein availability. This could impact pregnancy in two ways 1) significantly lower production of anti-inflammatory steroids since these are derived from cholesterol and 2) placental lack of intracellular molecules such as HDL3 directly increases oxidative stress. Logically, when these two effects combine, other antioxidant and anti-inflammatory pathways may compensate, or become increasingly overwhelmed.
In part, functional placental changes may be mediated by apoptotic signaling. Previous research has shown that oxidative stress tends to cause localized apoptosis in the labyrinth zone of the murine placenta [34]. Our study both supports and expands upon these findings, since we found higher apoptosis in both IVF and ICSI placentas concurrent to oxidative stress and inflammation. Additionally, degraded DNA (apoptosis) detected by TUNEL seems to be mirrored by generalized intracellular RNA degradation in ART placentas.
Despite higher apoptosis, decreased lipid accumulation and lower antioxidant enzyme capacity in ART placentas (particularly ICSI), significantly elevated levels of reactive oxygen species were not observed. The fluorimetric method employed here is more sensitive than other methods (such as thiobarbituric reactive substances) so our findings are unlikely to through low method sensitivity. Rather, since all tissues were collected, frozen, thawed, homogenized and then used, we propose that the increases in tissue damage from these processes were of sufficient magnitude to overcome [any] constitutive differences in ROS levels.
The BD62F1 mouse strain was used for normal mating, and as oocyte and sperm donors for ART while the CD-1 strain was used as surrogate mothers for ART. Therefore, it cannot be excluded that the maternal environment of the pregnancies (BD62F1 mothers for normally mated vs. CD1 mothers for IVF and ICSI) is responsible for differences observed. However, we do not expect that this is the major driving factor in our results because we also noted the differences between the two ART methods, with more invasive ICSI being worse. Moreover, with the exception of the decidua; the placenta is an organ of fetal origin and all of the fetuses were the same genetic background. Finally, the endocrinology of pregnancy as well as inflammatory and oxidative stress responses tend to be very tightly regulated within animal families (even across species) due to the requirements for maintaining the pregnant state as well as fetal growth and development. Hence we believe the underlying cause for our results is ART and not differing genetic background of mothers.
Finally, although the combined network of antioxidant defenses is important for “generalized” oxidative stress, inhibition of individual enzymes can also be cause for concern. Studies using SOD knockout mice have demonstrated a wide range of pathological symptoms including muscle loss, cataracts and retinopathies, and the development of hepatocarcinomas later in life [35#x2013;38]. Additionally, compromised SOD and GPx activities are part of the pathological process in human pre-eclampsia, which is also associated with oxidative stress and negative reproductive outcomes [38, 39]. Therefore, the marked inhibition of placental SOD observed herein may be a critical factor for negative outcomes such as pre-term birth, neonatal retinopathy and pediatric cancers that have greater incidence in ART pregnancies.
Here we provide the first evidence for dysregulation of placental and fetal antioxidant defenses by ART. Lower capacity of the placental antioxidant network was accompanied by lower quantities of essential lipids, increased pro-inflammatory signaling, higher levels of apoptosis, and damage to intracellular nucleotides. We are therefore proposing that excessive placental inflammation and oxidative stress may be a critical factor in the higher incidence of pre-term birth, low birth weight, pediatric imprinting disorders and cancers in ART. Notably, these studies were performed at term precisely when oxidative stress arises (perhaps as early as and implantation) remains unknown. While “oxidative stress” and “inflammation” are broad terms; the antioxidant enzymes #x2013; particularly SOD, as well as the IL-6 inflammatory pathway appear to be good candidates for further study into improving ART outcomes.
Acknowledgements
We thank Dr. Diane Wallace-Taylor for allowing us to use her bright-field microscope and image capture system. We also acknowledge Miyoko Bellinger from the JABSOM Histology core for outstanding sectioning of tissues.
Support: The study was supported by NIH HD058059 and P20RR024206 (project 2) grants to M.A.W. and NIH RR024206 (Project 4) to A.C.C.
Footnotes
Authorship Contributions: Research design: Collier, Ward Conducted experiments: Raunig, Collier, Yamauchi Performed data analysis: Raunig, Collier Wrote or contributed to the writing of the manuscript: Raunig, Ward and Collier Other: Collier and Ward acquired funding for this research
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have nothing to declare.
Abbreviations: assisted reproduction technologies: ART, catalase: CAT, intracytoplasmic sperm injection: ICSI, in vitro fertilization: IVF, glutathione peroxidase: GPx, and glutathione reductase: GR, glutathione-S-transferase: GST, superoxide dismutase: SOD, thioredoxin reductase: TrxR, xanthine oxidase: XO.
References
- [1].Allen VM, Wilson RD, Cheung A. Pregnancy outcomes after assisted reproductive technology. J Obstet Gynaecol Can. 2006;28:220–50. doi: 10.1016/S1701-2163(16)32112-0. [DOI] [PubMed] [Google Scholar]
- [2].Buckett WM, Chian RC, Holzer H, Dean N, Usher R, Tan SL. Obstetric outcomes and congenital abnormalities after in vitro maturation, in vitro fertilization, and intracytoplasmic sperm injection. Obstet Gynecol. 2007;110:885–91. doi: 10.1097/01.AOG.0000284627.38540.80. [DOI] [PubMed] [Google Scholar]
- [3].Kallen B, Finnstrom O, Nygren KG, Olausson PO. In vitro fertilization in Sweden: child morbidity including cancer risk. Fertil Steril. 2005;84:605–10. doi: 10.1016/j.fertnstert.2005.03.035. [DOI] [PubMed] [Google Scholar]
- [4].Shevell T, Malone FD, Vidaver J, Porter TF, Luthy DA, Comstock CH, et al. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol. 2005;106:1039–45. doi: 10.1097/01.AOG.0000183593.24583.7c. [DOI] [PubMed] [Google Scholar]
- [5].Delle Piane L, Lin W, Liu X, Donjacour A, Minasi P, Revelli A, Maltepe E, Rinaudo PF. Effect of the method of conception and embryo transfer procedure on midgestation placenta and fetal development in an IVF mouse model. Hum Reprod. 2010;25:2039–46. doi: 10.1093/humrep/deq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Thuan NV, Wakayama S, Kishigami S, Ohta H, Hikichi T, Mizutani E, et al. Injection of somatic cell cytoplasm into oocytes before intracytoplasmic sperm injection impairs full-term development and increases placental weight in mice. Biol Reprod. 2006;74:865–73. doi: 10.1095/biolreprod.105.047803. [DOI] [PubMed] [Google Scholar]
- [7].Collier AC, Miyagi SJ, Yamauchi Y, Ward MA. Assisted reproduction technologies impair placental steroid metabolism. J Steroid Biochem Mol Biol. 2009;116:21–8. doi: 10.1016/j.jsbmb.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Raunig JM, Yamauchi Y, Ward MA, Collier AC. Assisted Reproduction Technologies Alter Steroid Delivery to the Mouse Fetus During Pregnancy. J Steroid Biochem Mol Biol. 2011;126:26–34. doi: 10.1016/j.jsbmb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wakayama T, Whittingham DG, Yanagimachi R. Production of normal offspring from mouse oocytes injected with spermatozoa cryopreserved with or without cryoprotection. J Reprod Fertil. 1998;112:11–7. doi: 10.1530/jrf.0.1120011. [DOI] [PubMed] [Google Scholar]
- [10].Romundstad LB, Romundstad PR, Sunde A, von During V, Skjaerven R, Vatten LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod. 2006;21:2353–8. doi: 10.1093/humrep/del153. [DOI] [PubMed] [Google Scholar]
- [11].Gavriil P, Jauniaux E, Leroy F. Pathologic examination of placentas from singleton and twin pregnancies obtained after in vitro fertilization and embryo transfer. Pediatr Pathol. 1993;13:453–62. doi: 10.3109/15513819309048235. [DOI] [PubMed] [Google Scholar]
- [12].Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–48. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- [13].Murtha AP, Feng L, Yonish B, Leppert PC, Schomberg DW. Progesterone protects fetal chorion and maternal decidua cells from calcium-induced death. Am J Obstet Gynecol. 2007;196:257, e1–5. doi: 10.1016/j.ajog.2007.01.007. [DOI] [PubMed] [Google Scholar]
- [14].Schwartz N, Xue X, Elovitz MA, Dowling O, Metz CN. Progesterone suppresses the fetal inflammatory response ex vivo. Am J Obstet Gynecol. 2009;201:211, e1–9. doi: 10.1016/j.ajog.2009.05.012. [DOI] [PubMed] [Google Scholar]
- [15].Lopez Bernal A, Anderson AB, Turnbull AC. Cortisol:cortisone interconversion by human decidua in relation to parturition: effect of tissue manipulation on 11 beta-hydroxysteroid dehydrogenase activity. J Endocrinol. 1982;93:141–9. doi: 10.1677/joe.0.0930141. [DOI] [PubMed] [Google Scholar]
- [16].Anderson AB, Flint AP, Turnbull AC. Mechanism of action of glucocorticoids in induction of ovine parturition: effect on placental steroid metabolism. J Endocrinol. 1975;66:61–70. [PubMed] [Google Scholar]
- [17].Miller B. Effects of ovarian hormones on foetal and placental growth in the mouse. Austr J Biol Sci. 1978;31:641–8. doi: 10.1071/bi9780641. [DOI] [PubMed] [Google Scholar]
- [18].Mucci LA, Lagiou P, Tamimi RM, Hsieh CC, Adami HO, Trichopoulos D. Pregnancy estriol, estradiol, progesterone and prolactin in relation to birth weight and other birth size variables (United States) Cancer Causes Control. 2003;14:311–8. doi: 10.1023/a:1023966813330. [DOI] [PubMed] [Google Scholar]
- [19].Mark PJ, Smith JT, Waddell BJ. Placental and fetal growth retardation following partial progesterone withdrawal in rat pregnancy. Placenta. 2006;27:208–14. doi: 10.1016/j.placenta.2005.01.004. [DOI] [PubMed] [Google Scholar]
- [20].Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- [21].Greenspan P, Mayer EP, Fowler SD. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985;100:965–73. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].LeBel CP, Ali SF, McKee M, Bondy SC. Organometal-induced increases in oxygen reactive species: the potential of 2',7'-dichlorofluorescin diacetate as an index of neurotoxic damage. Toxicol Appl Pharmacol. 1990;104:17–24. doi: 10.1016/0041-008x(90)90278-3. [DOI] [PubMed] [Google Scholar]
- [23].Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- [24].Gonzalez P, Tunon MJ, Manrique V, Garcia-Pardo LA, Gonzalez J. Changes in hepatic cytosolic glutathione S-transferase enzymes induced by clotrimazole treatment in rats. Clin Exp Pharmacol Physiol. 1989;16:867–71. doi: 10.1111/j.1440-1681.1989.tb01526.x. [DOI] [PubMed] [Google Scholar]
- [25].Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- [26].Rock J, Pincus G, Garcia C, R. Effects of Certain 19-Nor Steroids on the Normal Human Menstrual Cycle. Science. 1956;124:891–3. doi: 10.1126/science.124.3227.891. [DOI] [PubMed] [Google Scholar]
- [27].Zhang Y, Zhao W, Jiang Y, Zhang R, Wang J, Li C, Zhao H, Gao L, Cui Y, Zhou Z, Sha J, Liu J, Wang L. Ultrastructural Study of Human Placentae from Women Subjected to Assisted Reproductive Technology Treatments. Biol Reprod. 2011 doi: 10.1095/biolreprod.110.090589. [epub ahead of print] Biol Reprod May 12, 2011 biolreprod.110.090589. [DOI] [PubMed] [Google Scholar]
- [28].Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T, Lengyel G, Mann GE, Pamplona R, Poli G, Portero-Otin M, Riahi Y, Salvayre R, Sasson S, Serrano J, Shamni O, Siems W, Siow RC, Wiswedel I, Zarkovic K, Zarkovic N. Pathological aspects of lipid peroxidation. Free Radical Research. 2010;44:1125–71. doi: 10.3109/10715762.2010.498478. [DOI] [PubMed] [Google Scholar]
- [29].Kontush A, Chapman MJ. Antiatherogenic function of HDL particle subpopulations: focus on antioxidative activities. Current Opinion in Lipidology. 2010;21:312–8. doi: 10.1097/MOL.0b013e32833bcdc1. [DOI] [PubMed] [Google Scholar]
- [30].Farese R, Jr, Ruland SL, Flynn LM, Stokowski RP, Young SG. Knockout of the mouse apolipoprotein B genes results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc Nat Acad Sci, USA. 1995;92:1774–8. doi: 10.1073/pnas.92.5.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Herz J, Farese RV. The LDL receptor gene family, apolipoprotein B and cholesterol in embryonic development. J Nutr. 1999;129:473S–5S. doi: 10.1093/jn/129.2.473S. [DOI] [PubMed] [Google Scholar]
- [32].Raabe M, Flynn LM, Zlot CH, Wong JS, Véniant MM, Hamilton RL, Young SG. Knockout of the abetalipoproteinemia gene in mice - reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc Nat Acad Sci, USA. 1998;95:8686–91. doi: 10.1073/pnas.95.15.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Woollett L. Maternal cholesterol in fetal development: transport of cholesterol from the maternal to the fetal circulation. Am J Clin Nutr. 2005;82:1155–61. doi: 10.1093/ajcn/82.6.1155. [DOI] [PubMed] [Google Scholar]
- [34].Burdon C, Mann C, Cindrova-Davies T, Ferguson-Smith AC, Burton GJ. Oxidative stress and the induction of cyclooxygenase enzymes and apoptosis in the murine placenta. Placenta. 2007;28:724–33. doi: 10.1016/j.placenta.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–81. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- [36].Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–80. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- [37].Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- [38].Sentman ML, Granstrom M, Jakobson H, Reaume A, Basu S, Marklund SL. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem. 2006;281:6904–9. doi: 10.1074/jbc.M510764200. [DOI] [PubMed] [Google Scholar]
- [39].Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertens Pregnancy. 2002;21:205–23. doi: 10.1081/PRG-120015848. [DOI] [PubMed] [Google Scholar]