TG2 ablation affects DC maturation and functions leading to a decreased pro-inflammatory response upon endotoxic shock stimulation.
Keywords: sepsis, human and mouse model
Abstract
DCs play an essential role in the endotoxic shock, and their profound depletion occurs in septic patients and septic mice. TG2−/− mice are more resistant to the endotoxic shock induced by LPS. Here, we studied the cellular and molecular basis of this effect, analyzing the role of the enzyme in DC maturation and function. We show that TG2 is up-regulated drastically during the final, functional maturation of DCs consequent to LPS treatment. In keeping with this finding, the inhibition of the enzyme cross-linking activity determines the impairment of DC function highlighted by wide phenotypic changes associated with a reduced production of cytokines (IL-10, IL-12) after LPS treatment and a lower ability to induce IFN-γ production by naïve T cells. The in vivo analysis of DCs obtained from TG2−/− mice confirmed that the enzyme ablation leads to an impairment of DC maturation and their reduced responsiveness to LPS treatment. In fact, a marked decrease in DC death, TLR4 down-regulation, and impaired up-regulation of MHCII and CD86 were observed in TG2−/− mice. Taken together, these data suggest that TG2 plays an important role in regulating the response of DCs to LPS and could be a candidate target for treating endotoxin-induced sepsis.
Introduction
DCs are professional APCs that play a dominant role in the initiation and regulation of immune response [1, 2]. Compared with other APCs, DCs have the unique capacity to stimulate naïve T cells, driving them into distinct classes of effectors cells. Encounter of antigen, e.g., from invading pathogens, causes DC activation, leading to their migration to peripheral lymph nodes, as well as their maturation. Mature DCs express high levels of MHCI, and MHCII and costimulatory molecules such as CD86, CD80, and CD40, which allows them to present antigen and activate T cells. In addition, during maturation, DCs secrete cytokines, which polarize Th cells toward Th1 or Th2, depending on the type of the secreted cytokines [1, 2]. The DC-derived cytokine IL-12 favors the differentiation of Th1 cells and is important for the development of immunity against intracellular bacterial infections. In contrast, DC-derived IL-10 promotes the polarization of Th cells toward Th2, which mediates immunity against extracellular parasites [3]. DCs, which lack high levels of costimulatory molecules and/or do not secrete proinflammatory cytokines, are involved in tolerance induction. In this case, tolerance is mediated through activation of Tregs or through induction of T cell apoptosis [4]. Thus, depending on the pattern of costimulatory molecules and secreted cytokines, DCs determine the fate of the immune response [5]. Defects in this maturation process may lead to immune suppression or autoimmunity. A number of studies indicate that sepsis causes marked immune suppression. It has been reported that during sepsis, depletion of DC takes place in various lymphoid and nonlymphoid tissues in patients [6] as well as in septic mice [7, 8]. Data obtained in mice indicate that sepsis produces divergent, functional changes in splenic and peritoneal DC populations [9]. Other studies have shown that in mice, splenic DCs acquire a state of aberrant responsiveness to bacterial stimuli, increase their expression of CD40 and CD86, but simultaneously, develop an impaired capacity to secrete IL-12 and to drive Th cell proliferation [10]. However, the mechanisms that underlie the development of functional defects of DCs during sepsis are not yet understood completely.
TG2 is a multifunctional enzyme involved in important biological processes, including cell death, signaling, cytoskeleton rearrangements, and extracellular matrix stabilization (for a complete review, see ref. [11]). TG2 displays enzymatic, G-protein, and nonenzymatic biological functions; it catalyzes post-translational modifications of proteins, such as protein–protein cross-linking, incorporation of amines and glutamine deamidation. TG2 can also act as a G-protein and as a protein disulfide isomerase [12,13,14,15]. As a result of these functions, aberrant activation of TG2 or deregulation of its function(s) plays an important role in the pathogenesis of a variety of human autoimmune, inflammatory, and degenerative diseases, such as celiac disease, diabetes, neurodegenerative diseases, multiple sclerosis, and rheumatoid arthritis (reviewed in ref. [16]). TG2−/− mice are prone to develop inflammatory pathologies. Furthermore, we have shown that these mice have an impaired capacity to clear apoptotic cells, and this defect is reflected in deregulated, inflammatory cytokine production by macrophages [17, 18].
As far as septic shock is concerned, TG2 expression has been reported to be induced by LPS in several tissues and organs [19,20,21]. We have demonstrated previously that the ablation of TG2 confers resistance to LPS-induced septic shock in mice, associated to the capacity to restore the initial equilibrium of circulating cytokines and proinflammatory mediators [22]. Based on these data, the objective of the present study was to get further insight into the cellular mechanisms leading to the improper regulation of the inflammatory response involved in sepsis, focusing our attention on the possible role exerted by TG2 on DC function. It is well known that the expression of TG2 increases drastically during monocyte differentiation into macrophages [23, 24], but little is known about its role in DCs. Therefore, we performed an in vitro and in vivo study to determine whether TG2 is involved in DC maturation and function.
MATERIALS AND METHODS
Mice
Male mice, 6–8 weeks old, were used in all experiments. Animals were maintained in the pathogen-free animal facility of the University of Rome “Tor Vergata” (Rome, Italy) under the guidelines and ethically approved protocols. WT C57BL/6 mice and TG2−/− mice on pure C57BL/6 background were obtained from the laboratory of G. Melino (University of Rome “Tor Vergata”).
mAb and reagents
The following mAb were purchased from BD Biosciences (San Jose, CA, USA) for flow cytometry experiments: FITC-conjugated anti-mouse CD86 mAb, PE-conjugated anti-mouse TLR4/MD-2 mAb, PE-conjugated anti-mouse MHCII mAb (I-Ab), allophycocyanin-conjugated anti-mouse CD11c mAb, and rat anti-mouse FcR mAb (2.4G2), ApoAlert Annexin V-FITC apoptosis kit, FITC-conjugated anti-human CD1a mAb, FITC-conjugated anti-human CD80 mAb, PE-conjugated anti-human CD11c mAb, PE-conjugated anti-human CD86 mAb, PerCP-conjugated anti-human MHCII mAb (HLA-DR), allophycocyanin-conjugated anti-human MHCI mAb (HLA-A, -B, and -C), allophycocyanin-conjugated anti-human CD14 mAb, and purified anti-human CCR7 mAb.
Human rIL-4 and human rGM-CSF were purchased from Peprotech (Rocky Hill, NJ, USA). LPS was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Mouse anti-human mAb TG2 (clones CUB 7402+TG100) was purchased from NeoMarkers (Fremont, CA, USA); mouse anti-human GAPDH mAb (6C5) was purchased from Calbiochem (Darmstadt, Germany). CD14 microbeads for human monocyte isolation were purchased from Miltenyi Biotec (Germany).
DC differentiation and cultures
Human PBMCs were isolated from healthy adult donors by density gradient centrifugation using Lympholite-H (Cedarlane, Canada). Monocytes were positively separated by anti-CD14 magnetic beads (MACS, Miltenyi Biotec), according to the manufacturer’s specifications. MoDCs were obtained by culturing monocytes at 37°C in 5% CO2 at a density of 1 × 106 cells/ml in RPMI-1640 medium with 10% FCS with 10 mM Hepes, 2 mM L-glutamine, 2 mM penicillin, 50 μg/ml streptomycin, 50 ng/ml GM-CSF, and IL-4 for 5 days. MoDCs were matured for 24 h by the addition of LPS (200 ng/ml). TG2 cross-linking activity was inhibited by addition of different concentrations (10, 50, and 100 μM) of the KCC009 inhibitor (compound 1b; see refs. [25,26,27,28]) on Day 0 of culture.
In vivo LPS treatment of murine DCs
WT and TG2−/− mice were injected i.p. with 40 mg/kg LPS solubilized in PBS. Control animals were injected with the same volume of PBS. Eighteen to 20 h after, the animals were killed, and total splenocytes were isolated. DC phenotype was analyzed by flow cytometry on gated, CD11c-positive cells.
Flow cytometry
Surface immunophenotyping of mouse cells was performed by preincubation with rat anti-mouse FcR mAb 2.4G2 for 15 min and then washed and stained with fluorochrome-conjugated mAb for 15 min at 4°C. After washing, cells were fixed in 1% PFA and acquired using a FACSCalibur cytometer (Becton Dickinson Italia S.p.A. Italy).
Human cells were stained with fluorochrome-conjugated mAb for 15 min at 4°C and then washed and fixed in 1% PFA. Acquisition was performed using a FACSCalibur cytometer. Multiparameter data acquisition and analysis were performed with CellQuest software (BD Immunocytometry Systems, San Jose, CA, USA).
Naive T cell polarization assay
The ability of MoDCs to stimulate and polarize naive T cells was evaluated. Allogeneic, naive CD4+ T cells were isolated from PBMCs using the CD4 naive T cell isolation kit (Miltenyi Biotec), according to the manufacturer’s specifications. After extensive washing, MoDCs (2×105) were cultured with allogeneic T cells (8×105) in 24-well plates. After 9 days, culture supernatants were collected and frozen at −80°C.
Cytokine assay
Supernatants of MoDCs (1×106/ml), derived in different conditions, as well as the supernatants from MoDC-T cell cocultures, were collected and stored at −80°C. IL-10 (limit of sensitivity, <5 pg), IL-12 (p70 subunit; limit of sensitivity, <5 pg), and IFN-γ (limit of sensitivity, <5 pg) levels were determined by ELISA kits (Pierce Endogen, Rockford, IL, USA), according to the manufacturer’s specifications. Results are expressed as pg/ml and reported as means.
Western blotting
MoDCs were pelleted and lysed using ice-cold cell lytic buffer (Sigma-Aldrich). Protein extracts were heated for 10 min at 97°C in Laemmli buffer and loaded on a 10% SDS polyacrylamide gel, which was run in Tris-glycine buffer at 100 V and transferred to the nitrocellulose membrane (Whatman, Germany). The membranes were incubated with 5% milk in PBS-Tween to prevent nonspecific binding of the mAb. The membranes were then incubated with anti-TG2 and anti-GAPDH primary antibodies overnight at 4°C. After several washes with PBS-Tween, membranes were incubated with the appropriate secondary antibodies for 1 h. Following a final wash step, protein signal was detected by chemiluminescence (Amersham/GE HealthCare, Buckinghamshire, UK).
Statistical analysis
Statistical analysis was conducted using a nonparametric, unpaired test. P < 0.05 values were considered statistically significant. GraphPad Prism, Version 5.00 for Windows (GraphPad Software, San Diego, CA, USA), was used to perform the analysis.
RESULTS
TG2 expression during DC differentiation
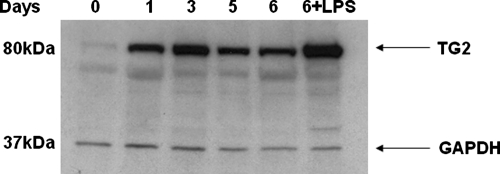
To determine whether TG2 is involved in the differentiation of DCs, we first analyzed its protein levels during the human monocyte differentiation into MoDC. To this aim, blood-derived human monocytes were grown in the presence of GM-CSF and IL-4 for 5 days, followed by another day in the presence of the maturation stimulus (LPS), and a time-course analysis of TG2 protein levels was carried out by Western blot analysis. The data reported in Figure 1 show that monocytes express low amounts of TG2, but the enzyme levels keep increasing significantly during the MoDC differentiation. Interestingly, we observed that mature DCs express higher TG2 levels compared with the immature ones, thus suggesting a possible role of TG2 in MoDC differentiation process and functions.
Figure 1.
Western blot analysis of the TG2 expression in differentiating MoDCs. Human DCs were generated from monocytes for 5 days in culture with GM-CSF, and IL-4 and LPS treatment was performed on Day 5 for 24 h. Cells were collected on Days 0 (monocytes), 1, 3, 5, and 6 (control and LPS-treated) and lysed, and total protein extract was analyzed by Western blot for TG2 expression. Anti-GAPDH mAb was used as a control of the total amount of the collected protein.
Effect of TG2 inhibition on human MoDC maturation and function
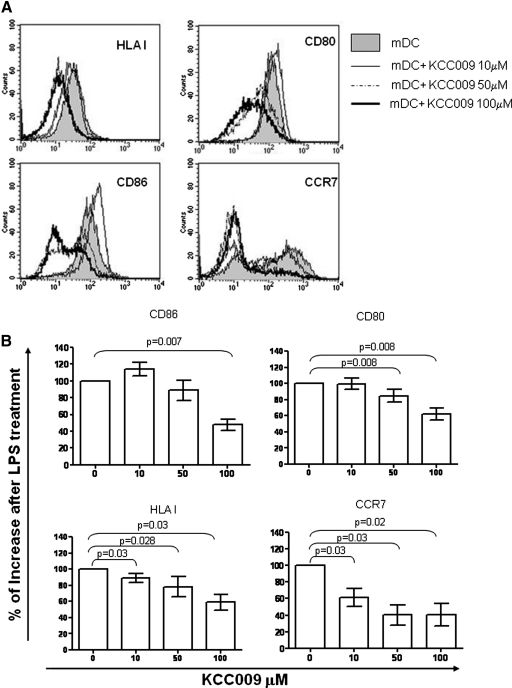
To determine the role of TG2 in the differentiation of MoDC, we used a specific TG2 cross-linking activity inhibitor, KCC009. To this aim, we differentiated MoDCs in the presence of an increasing concentration of KCC009 for 5 days, followed by another day in the presence of the maturation stimulus, LPS. First, we evaluated cell viability by Trypan blue exclusion. The number of live cells was high (>99%; data not shown) in the control cells as well as in presence of KCC009, indicating that KCC009 had no toxic effects on DC viability. Then, we tested whether the inhibition of TG2 plays a role in MoDC differentiation and maturation. We found that the inhibition of TG2 cross-linking activity did not alter the capacity of monocytes to differentiate into DCs, as they down-regulate the CD14 molecule and express higher levels of CD1a. In addition, the TG2-inhibited MoDCs display similar levels of HLAI and - II and costimulatory molecules compared with untreated controls (data not shown). However, after LPS treatment, TG2-inhibited MoDCs present a dose-dependent down-modulation of CD80, CD86, HLAI, and CCR7 (Fig. 2, A and B), suggesting that TG2 cross-linking activity could be involved in the MoDC maturation process.
Figure 2.
Phenotypic analysis of MoDCs treated with KCC009, a small-molecule TG2 inhibitor. Human DCs were generated from monocytes (mDC) after 5 days of culture with GM-CSF and IL-4 and in the presence of different concentrations of KCC009. Then, cells were treated with LPS for 24 h, and mature DC (mDC) phenotype was analyzed. (A) The expression of the indicated molecules (as histograms) of a representative experiment. (B) The effect of KCC009 on DC maturation obtained from all of the experiments is represented. The results are shown as percentage of the increase of molecule expression in the LPS-treated DCs upon treatment with different concentrations of KCC009.
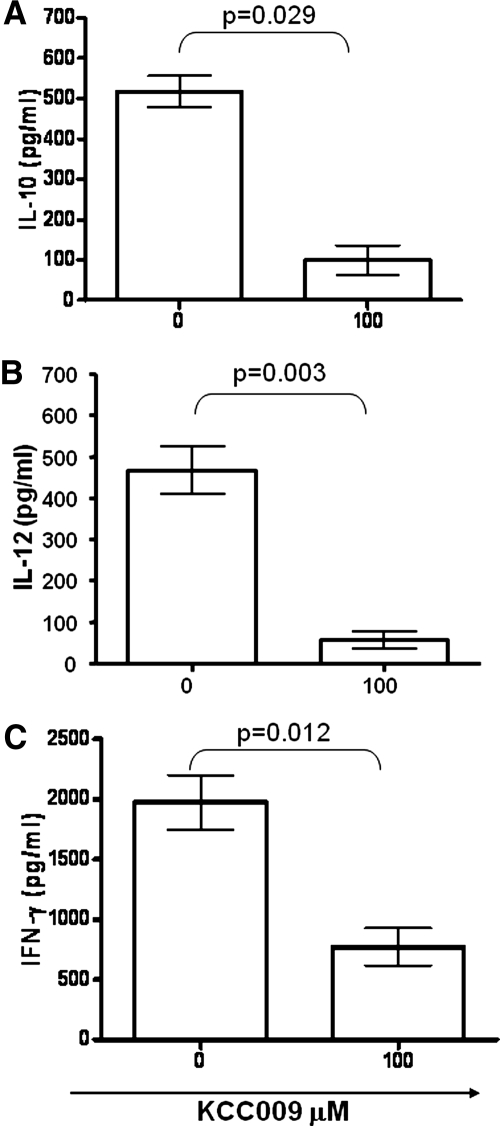
Upon proper stimulation, DCs are able to secrete IL-10 and IL-12, which play a central role in the regulation of the immune response. We therefore evaluated whether the TG2 inhibiton by KCC009 was able to prevent the release of IL-10 and IL-12 in the supernatants of MoDC after 24 h upon LPS treatment. Indeed, the pretreatment of MoDC with the TG2 inhibitor strongly impaired the secretion of IL-10 (Fig. 3A) and IL-12 (Fig. 3B), indicating a regulatory role played by the enzyme on DC functions.
Figure 3.
IL-10 and IL-12p70 production by MoDCs and IFN-γ accumulation in the supernatants of naïve CD4 T cells cultured with allogeneic MoDCs. Human DCs were generated from monocytes after 5 days culture with GM-CSF and IL-4 and in the presence of 100 μM KCC009, a TG2 inhibitor. Cells were treated with LPS for a further 24 h, and then, supernatants were collected and samples stored at −80°C. The levels of IL-10 (A) and IL-12 (B) were tested by ELISA. Results are expressed as mean values (+sem) of six independent experiments. Naïve CD4 T cells (1×106) were cultured with heterologous MoDCs differentiated in the presence of KCC009 (100 μM); the supernatants were collected after 9 days and stored at −80°C. The levels of IFN-γ (C) were tested by ELISA. Data are expressed as mean values (+sem) of cytokine levels of four independent experiments.
KCC009-treated DCs are not able to polarize naïve CD4+ T cells
MoDCs play a major role in directing the immune response, having a unique capacity to stimulate naïve T lymphocytes, driving them into distinct classes of effector cells (Th1, Th2, and Treg). Considering that TG2 inhibition significantly reduces the production of regulatory cytokines by MoDCs, we wondered whether the treatment with KCC009 alters their capacity to polarize the immune response. To address this issue, we examined the nature of the primary, allogeneic CD4 naïve T cell response induced by KCC009-treated MoDCs after 9 days of coculture. Untreated and KCC009-treated DCs induced similar levels of proliferation of T cells (data not shown). T cell-derived IFN-γ was measured by ELISA. As expected, T cells cocultured with allogeneic, mature MoDCs produced high amount of IFN-γ. In contrast, T cells cocultured with allogeneic MoDCs, differentiated in the presence of KCC009, showed a significant decrease in the IFN-γ release (Fig. 3C), indicating that the TG2 inhibition also impaired the MoDC capacity to polarize the Th1 immune response. However, when IL-4 secretion by T cells in response to control and KCC009-treated DCs was tested, we did not find detectable levels of IL-4, suggesting a complete impairment of DC-induced T cell polarization.
Effect of TG2 ablation on mouse DC functions
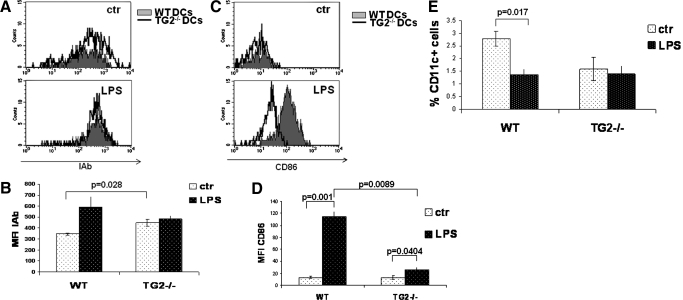
The results described above indicate an important role for TG2 in the maturation and functions of human DCs. Our previous work demonstrated that TG2 ablation leads to partial resistance to experimental sepsis, reflecting a reduction of tissue injury and a better homeostasis of the proinflammatory mediators [18]. These findings raised the question of whether the TG2-ablated DC population could play a role in this phenomenon. To this aim, we performed the characterization of TG2−/− DCs upon in vivo stimulation with LPS. We injected LPS in the peritoneal cavity of WT and TG2−/− mice and analyzed a splenic DC phenotype. Our results showed that at the steady-state, TG2−/− DCs express higher levels of MHCII molecule on their surface compared with WT DCs (Fig. 4A). However, upon LPS stimulation, DCs from TG2−/− mice have a lower ability to up-regulate MHCII and the costimulatory molecule CD86 compared with WT (Fig. 4, B and D, respectively). In addition, we measured the number of splenic DCs before and after LPS treatment, and we found that in WT mice, the number of spleen CD11c-positive cells decreased after LPS treatment (∼35%), as expected [9]. In contrast, in TG2−/− mice, the percentage of spleen DCs did not change (Fig. 4E).
Figure 4.
Effect of TG2 ablation on the phenotype and number of spleen DCs in response to in vivo LPS injection. WT and TG2−/− C57BL/6 mice were injected with LPS (40 mg/kg) i.p. After 18–20 h, the total cell population of spleen was isolated, and phenotype analysis of DCs was performed for expression of I-Ab, CD86, and CD11c. (A) Two representative dot histograms [control (ctr) and LPS] of the expression of I-Ab on gated, CD11c-positive splenic cells (WT, filled histograms; TG2−/−, empty histograms). (B) The mean values [expressed as mean fluorescence intensity (MFI)] for the I-Ab expression (control, white-dotted bars; LPS, black-dotted bars) in WT (three mice) and TG−/− (three mice). (C) Two representative dot histograms (control and LPS) of the expression of CD86 from gated, CD11c-positive splenic cells (WT, filled histograms; TG2−/−, empty histograms). (D) The mean values (expressed as mean fluorescence intensity) for the CD86 expression (control, white-dotted bars; LPS, black-dotted bars) in WT (three mice) and TG−/− (three mice). (E) The percentage of the splenic, CD11c-positive cell population from WT (three mice) and TG−/− (three mice) is shown (control, white-dotted bars; LPS, black-dotted bars).
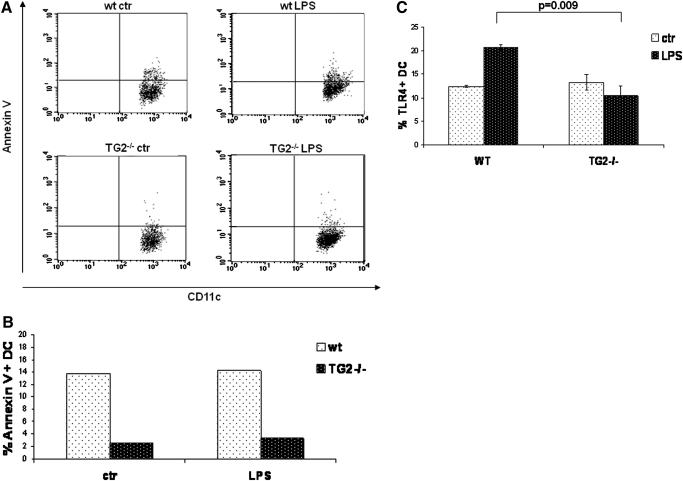
As TG2 is involved in the regulation of cell death [11], we analyzed the apoptosis levels in the DC population resident in spleen after in vivo LPS treatment. Figure 5, A and B, shows that the level of apoptosis in spleen DCs from TG2−/− mice is much lower before and after endotoxin challenge compared with WT. Similar results were obtained when ex vivo splenocytes were treated with LPS and apoptosis monitored at various time-points up to 18 h after challenge (data not shown).
Figure 5.
In vivo effect of LPS on apoptosis and TLR4 expression on spleen DCs from TG2−/−and WT mice. Annexin V and TLR4 expression were tested by flow cytometry. (A) Two representative dot-plot graphs for the number of cells positive for annexin V are shown. (B) The percentage of annexin V-positive, splenic DCs from WT and TG2−/− control (white-dotted bars) and LPS-injected TG2 (black-dotted bars) mice from the same representative experiment. (C) The mean percentage of TLR4-positive, splenic DCs in WT and TG2−/− control mice (white-dotted bars) and LPS-injected TG2 (black-dotted bars), from three independent experiments.
Finally, we wondered whether the impaired response of DCs to LPS observed in TG2−/− mice could involve a defective TLR4 expression. Interestingly, WT and TG2−/− express similar levels of TLR4 under physiological conditions; by contrast, upon LPS injection, DCs from TG2−/− mice showed a lower number of TLR4-positive cells compared with WT (Fig. 5B), indicating an impaired TLR4 regulation.
DISCUSSION
Antigen presentation by DCs and their capacity to respond directly to an invading pathogen are the key factors in initiation as well as regulation of immune response [1]. As such, DCs are of great interest as targets for therapeutic strategies to protect against pathogens and stimulate immune response against cancer [29]. Moreover, the role of DCs in septic shock has been highlighted recently by the fact that these cells are depleted profoundly in septic mice and patients [6,7,8]. However, as a result of the complexity of interactions of DCs with other cells and microbes, the mechanisms triggering activation, maturation, migration, and tolerogenic properties of DCs are not yet clearly understood. Recent data published by our group indicated TG2 as a possible “player” in the molecular and cellular mechanisms leading to improper regulation of inflammatory response involved in sepsis [22]. TG2 is known to function in various cell locations and to play important roles in many processes, including cell death, cell movement, adhesion, and proliferation [11]. The defective expression of TG2 activity is associated with diverse disorders; for example, Huntington’s, Alzheimer’s, and Parkinson’s diseases are related to transamidation and celiac disease to deamidation. It has already been demonstrated that TG2 expression increases during monocyte transendothelial migration and differentiation into macrophages [30]. Thus, in the current study, we explored the potential role of TG2 in differentiation and function in human and mouse DC models. We found that TG2 increased as the human DC differentiation progressed. Moreover, when stimulated with LPS, human DCs up-regulated the levels of TG2 further, again suggesting a role in the DC functions. Our results showed that the inhibition of TG2 activity by KCC009 did not alter monocyte differentiation to phenotypically immature DCs. However, KCC009 did influence the ability of these DCs to mature when stimulated with LPS. This was reflected in the DC phenotype as well as in the cytokine production, indicating that the enzyme might have a role in the function of DC. It has been shown that β2-microglobulin is one of the substrates of TG2 [31], which could imply TG2 involvement in the assembly of the MHCI. In fact, upon synthesis, the MHCI heavy chain binds to the membrane-associated endoplasmic reticulum chaperone, calnexin. Upon dissociation from calnexin, the heavy chain binds to β2-microglobulin and is then incorporated into the peptide-loading complex [32]. The other constituents of the complex are the two subunits of the transporter associated with antigen processing (TAP1 and TAP2), the transmembrane glycoprotein tapasin, the soluble chaperone calreticulin, and the thiol oxidoreductase ERp57. Interestingly, TG2 is known to interact with calreticulin [33] and to cross-link β2-microglobulin [31]; thus, it seems plausible to hypothesize that the enzyme could play a role in the proper assembly of the MHCI complex.
It is well known that DCs are able to polarize naive T cells toward IFN-γ-producing Th1 or IL-4-producing Th2 cells, depending on the DC subtypes and microenvironmental conditions [34]. Our results showed a defect in T cell polarization when DCs were treated with the TG2 inhibitor; in fact, DCs were unable to produce a proper Th1 response in coculture with allogeneic T lymphocytes. On the basis of these findings, we might suggest that cross-linking activity of TG2 is involved in the final maturation and function of DCs, thus contributing to the development of sepsis pathogenesis. Our previous data showed that the TG2 expression increases during endotoxemia in mice and that a better homeostasis of the proinflammatory mediators was observed in TG2−/− mice, suggesting that TG2 could be responsible for a vicious inflammatory cycle [35]. To substantiate this hypothesis further, we studied the role of TG2 in DC functions in a TG2−/− mouse model. We found that although splenic DCs showed higher expression of MHCII in TG2−/− compared with WT mice, the capacity of these cells to respond to LPS in vivo was reduced in the absence of TG2. In particular, DCs from TG2−/− mice were not able to up-regulate MHCII and CD86 upon LPS stimulation. Interestingly, we found that LPS induced a splenic DC depletion in WT mice that was not detected in TG2−/− mice. In agreement, DC survival was demonstrated to be pivotal in the resistance to lethal endotoxic shock [35]. One explanation can be the reduced susceptibility of TG2−/− DCs to undergo apoptosis upon LPS challenge. In fact, we found reduced apoptosis levels in TG2−/− DCs before and after treatment with LPS. It is well known that TG2−/− can be externalized and by interacting with the extracellular matrix, can regulate the adhesive properties of various cells [36]. Thus, we cannot exclude that the absence of TG2, normally localized on DC plasma membranes [37], could be responsible for the preservation of DC number.
Altogether, these data indicate that TG2 ablation markedly affects DC maturation and functions, thereby determining a decreased capacity of these cells to produce a proinflammatory response upon endotoxic shock stimulation. An important reason for DC unresponsiveness might rely on the expression of the LPS receptor [38]. DCs and other cells react to bacterial motifs, such as LPS, via TLR4, and consecutively up-regulate other members of the Toll-like family (TRL2 and TLR9) [38, 39]. The TLR4-MD-2 complex resides on the plasma membrane [39], and LPS induces its clustering, a mechanism critical for TLR4 signaling [38]. When we tested TG2−/− DCs for the expression of TLR receptors, indeed, we found that although in DCs from LPS-treated WT mice, an up-regulation of TLR4/MD-2 complex expression was induced, in the DCs from TG2-deficient animals, the receptor was not up-regulated. Such a phenomenon has been reported already in unresponsive animals upon LPS stimulation [40], confirming that the defect in TLR4 receptor expression detected in DCs obtained from TG2−/− mice could also be responsible for the impaired responsiveness of these cells to sepsis.
From all of these data, we can conclude that the expression of TG2 seems to play an important homeostatic function when differentiated DCs are challenged by a stressful antigenic stimulus that is reproduced in our experimental settings by LPS. Taken together, our results indicate a pleiotropic TG2 role in the maturation and survival of DCs, which might explain the impaired response to LPS stimulation that we reported in TG2−/− mice. Future studies should clarify by which mechanism(s) TG2 participates in the regulation of DC function in response to bacterial compounds. To achieve this result, the identification and characterization of the enzyme’s substrate protein profile in APCs are absolutely required. Finally, these findings highlight TG2 inhibition as a new target for the treatment of inflammatory processes associated with sepsis.
AUTHORSHIP
A. Sacchi and I. Matic contributed equally to this study by performing most of the experimental work and writing the manuscript. A. Rinaldi contributed by performing the experiments with human MoDCs. Prof. G. Melino provided the TG2−/− mice. Prof. C. Khosla provided the transglutaminase inhibitors and contributed to the experiments with human MoDCs. L. Falasca contributed by performing in vivo injections in the murine model system. Prof. M. Piacentini supervised all of the work performed.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Health of Italy “Ricerca Corrente” and “Ricerca Finalizzata,” AIRC, and Fondazione Telethon to M. P. and by the NIH (DK063158) to C. K. I. M. is supported by an early scientist fellowship provided by the EU Marie Curie grant “TRACKS.” The support of the EU grant “Apo-Sys” to M. P. is also acknowledged.
Footnotes
Abbreviations: DC=dendritic cell, MD-2=myeloid differentiation protein 2, MoDC=monocyte-derived dendritic cells, PFA=paraformaldehyde, TAP=transporter-associated protein, TG2=transglutaminase type 2, TG2−/−=transglutaminase type 2 knockout, Treg=regulatory T cell, WT=wild-type
References
- Liu Y. J., Kanzler H., Soumelis V., Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–589. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R. M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Kaliński P., Hilkens C. M., Wierenga E. A., Kapsenberg M. L. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Nussenzweig M. C. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M. B., Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. S., Tinsley K. W., Swanson P. E., Grayson M. H., Osborne D. F., Wagner T. H., Cobb J. P., Coopersmith C., Karl I. E. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M., Hotchkiss R. S., Karl I. E., Buchman T. G. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–253. doi: 10.1097/00024382-199704000-00002. [DOI] [PubMed] [Google Scholar]
- Scumpia P. O., McAuliffe P. F., O'Malley K. A., Ungaro R., Uchida T., Matsumoto T., Remick D. G., Clare-Salzler M. J., Moldawer L. L., Efron P. A. CD11+ dendritic cells are required for survival in murine polymicrobial sepsis. J Immunol. 2005;175:3282–3286. doi: 10.4049/jimmunol.175.5.3282. [DOI] [PubMed] [Google Scholar]
- Ding Y., Chung C. S., Newton S., Chen Y., Carlton S., Albina J. E., Ayala A. Polymirobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock. 2004;22:137–144. doi: 10.1097/01.shk.0000131194.80038.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flohé S. B., Agrawal H., Schmitz D., Gertz M., Flohé S., Schade F. U. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J Leukoc Biol. 2006;79:473–481. doi: 10.1189/jlb.0705413. [DOI] [PubMed] [Google Scholar]
- Piacentini M., Fesus L. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem Sci. 2002;27:534–539. doi: 10.1016/s0968-0004(02)02182-5. [DOI] [PubMed] [Google Scholar]
- Iismaa S. E., Wu M. J., Nanda N., Church W. B., Graham R. M. GTP binding and signaling by Gh/transglutaminase II involves distinct residues in a unique GTP-binding pocket. J Biol Chem. 2000;275:18259–18265. doi: 10.1074/jbc.M000583200. [DOI] [PubMed] [Google Scholar]
- Hasegawa G., Suwa M., Ichikawa Y., Ohtsuka T., Kumagai S., Kikuchi M., Sato Y., Saito Y. A novel function of tissue-type transglutaminase: protein disulphide isomerase. Biochem J. 2003;373:793–803. doi: 10.1042/BJ20021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Graham R. M. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Piacentini M., Amendola A., Ciccosanti F., Falasca L., Farrace M. G., Mastroberardino P. G., Nardacci R., Oliverio S., Piredda L., Rodolfo C., Autuori F. Type 2 transglutaminase and cell death. Prog Exp Tumor Res. 2005;38:58–74. doi: 10.1159/000084233. [DOI] [PubMed] [Google Scholar]
- Facchiano F., Facchiano A., Facchiano A. M. The role of transglutaminase-2 and its substrates in human diseases. Front Biosci. 2006;11:1758–1773. doi: 10.2741/1921. [DOI] [PubMed] [Google Scholar]
- Falasca L., Iadevaia V., Ciccosanti F., Melino G., Serafino A., Piacentini M. Transglutaminase type II is a key element in the regulation of the anti-inflammatory response elicited by apoptotic cell engulfment. J Immunol. 2005;174:7330–7340. doi: 10.4049/jimmunol.174.11.7330. [DOI] [PubMed] [Google Scholar]
- Tóth B., Garabuczi E., Sarang Z., Vereb G., Vámosi G., Aeschlimann D., Blaskó B., Bécsi B., Erdõdi F., Lacy-Hulbert A., Zhang A., Falasca L., Birge R. B., Balajthy Z., Melino G., Fésüs L., Szondy Z. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol. 2009;182:2084–2092. doi: 10.4049/jimmunol.0803444. [DOI] [PubMed] [Google Scholar]
- Leu R. W., Herriott M. J., Moore P. E., Orr G. R., Birckbichler P. J. Enhanced transglutaminase activity associated with macrophage activation Possible role in Fc-mediated phagocytosis. Exp Cell Res. 1982;141:191–199. doi: 10.1016/0014-4827(82)90081-7. [DOI] [PubMed] [Google Scholar]
- Bowness J. M., Tarr A. H. Increase in transglutaminase and its extracellular products in response to an inflammatory stimulus by lipopolysaccharide. Mol Cell Biochem. 1997;169:157–163. doi: 10.1023/a:1006846400478. [DOI] [PubMed] [Google Scholar]
- Park K-C., Chung K-C., Kim Y-S., Lee J., Joh T. H., Kim S-Y. Transglutaminase 2 induces nitric oxide synthesis in BV-2 microglia. Biochem Biophys Res Commun. 2004;323:1055–1062. doi: 10.1016/j.bbrc.2004.08.204. [DOI] [PubMed] [Google Scholar]
- Falasca L., Farrace M. G., Rinaldi A., Tuosto L., Melino G., Piacentini M. Transglutaminase type II is involved in the pathogenesis of endotoxic shock. J Immunol. 2008;180:2616–2624. doi: 10.4049/jimmunol.180.4.2616. [DOI] [PubMed] [Google Scholar]
- Murtaugh M. P., Mehta K., Johnson J., Myers M., Juliano R. L., Davies P. J. Induction of tissue transglutaminase in mouse peritoneal macrophages. J Biol Chem. 1983;258:11074–11081. [PubMed] [Google Scholar]
- Akimov S. S., Belkin A. Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fibronectin. Blood. 2001;98:1567–1576. doi: 10.1182/blood.v98.5.1567. [DOI] [PubMed] [Google Scholar]
- Choi K., Siegel M., Piper J. L., Yuan L., Cho E., Strnad P., Omary B., Rich K. M., Khosla C. Chemistry and biology of dihydroisoxazole derivatives: selective inhibitors of human transglutaminase 2. Chem Biol. 2005;12:469–475. doi: 10.1016/j.chembiol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Yuan L., Behdad A., Siegel M., Khosla C., Higashikubo R., Rich K. M. Tissue transgluaminase 2 expression in meningiomas. J Neurooncol. 2008;90:125–132. doi: 10.1007/s11060-008-9642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Siegel M., Choi K., Khosla C., Miller C. R., Jackson E. N., Piwnica-Worms D., Rich K. M. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthotopic glioblastomas to chemotherapy. Oncogene. 2007;26:2563–2573. doi: 10.1038/sj.onc.1210048. [DOI] [PubMed] [Google Scholar]
- Strnad P., Siegel M., Toivola D. M., Choi K., Kosek J. C., Khosla C., Omary M. B. Pharmacologic transglutaminase inhibition attenuates drug-primed liver hypertrophy but not Mallory body formation. FEBS Lett. 2006;580:2351–2357. doi: 10.1016/j.febslet.2006.03.051. [DOI] [PubMed] [Google Scholar]
- Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Ecker S., Lindecke A., Hatzmann W., Kaltschmidt C., Zänker K. S., Dittmar T. Alteration in the gene expression pattern of primary monocytes after adhesion to endothelial cells. Proc Natl Acad Sci USA. 2007;104:5539–5544. doi: 10.1073/pnas.0700732104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fésüs L., Falus A., Erdei A., Laki K. Human β 2-microglobulin is a substrate of tissue transglutaminase: polymerization in solution and on the cell surface. J Cell Biol. 1981;89:706–710. doi: 10.1083/jcb.89.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell P., Ackerman A. L., Giodini A., Peaper D. R., Wearsch P. A. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–157. doi: 10.1111/j.0105-2896.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- Feng J. F., Readon M., Yadav S. P., Im M. J. Calreticulin down-regulates both GTP binding and transglutaminase activities of transglutaminase II. Biochemistry. 1999;38:10743–10749. doi: 10.1021/bi9905009. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Kadowaki N., Rissoan M. C., Soumelis V. T cell activation and polarization by DC1 and DC2. Curr Top Microbiol Immunol. 2000;251:149–159. doi: 10.1007/978-3-642-57276-0_19. [DOI] [PubMed] [Google Scholar]
- Gautier E. L., Huby T., Saint-Charles F., Ouzilleau B., Chapman M. J., Lesnik P. Enhanced dendritic cell survival attenuates lipopolysaccharide-induced immunosuppression and increases resistance to lethal endotoxic shock. J Immunol. 2008;180:6941–6946. doi: 10.4049/jimmunol.180.10.6941. [DOI] [PubMed] [Google Scholar]
- Ráki M., Schjetne K. W., Stamnaes J., Molberg Ø., Jahnsen F. L., Issekutz T. B., Bogen B., Sollid L. M. Surface expression of transglutaminase 2 by dendritic cells and its potential role for uptake and presentation of gluten peptides to T cells. Scand J Immunol. 2007;65:213–220. doi: 10.1111/j.1365-3083.2006.01881.x. [DOI] [PubMed] [Google Scholar]
- Zemskov E. A., Janiak A., Hang J., Waghray A., Belkin A. M. The role of tissue transglutaminase in cell-matrix interactions. Front Biosci. 2006;11:1057–1076. doi: 10.2741/1863. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Akashi S., Nagafuku M., Ogata M., Iwakura Y., Akira S., Kitamura T., Kosugi A., Kimoto M., Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- Powers K. A., Szászi K., Khadaroo R. G., Tawadros P. S., Marshall J. C., Kapus A., Rotstein O. D. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J Exp Med. 2006;203:1951–1961. doi: 10.1084/jem.20060943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Cook J. A. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]