Abstract
Most organisms possess anti-predator adaptations to reduce their risk of being consumed, but little is known of the adaptations prey employ during vulnerable life-history transitions when predation pressures can be extreme. We demonstrate the use of a transition-specific anti-predator adaptation by coral reef fishes as they metamorphose from pelagic larvae to benthic juveniles, when over half are consumed within 48 h. Our field experiment shows that naturally settling damselfish use olfactory, and most likely innate, predator recognition to avoid settling to habitat patches manipulated to emit predator odour. Settlement to patches emitting predator odour was on average 24–43% less than to control patches. Evidence strongly suggests that this avoidance of sedentary and patchily distributed predators by nocturnal settlers will gain them a survival advantage, but also lead to non-lethal predator effects: the costs of exhibiting anti-predator adaptations. Transition-specific anti-predator adaptations, such as demonstrated here, may be widespread among organisms with complex life cycles and play an important role in prey population dynamics.
Keywords: life-history transition, metamorphosis, anti-predator strategy, mortality, coral reef fish, non-lethal effects
1. Introduction
Most prey face the omnipresent threat of being consumed by a predator and possess anti-predator adaptations to reduce this risk. These include behavioural, physiological, morphological and developmental responses to a predator's presence [1]. Many anti-predator adaptations are situation-specific, i.e. they work only in a given environment, for a certain type of predator, and require the prey to have specific capabilities. The changing nature of these factors throughout prey ontogeny means that prey require life-stage-specific anti-predator adaptations. This is particularly evident in the many taxa that undergo a rapid concurrent change in morphology and ecology as they transition from larvae to juveniles during metamorphosis [2], e.g. many insects, amphibians, marine invertebrates and fishes. The anti-predator mechanisms used either side of metamorphosis are relatively well known; however, there is scant evidence for adaptations used to mitigate mortality during the transition itself (but see [3,4]). This is somewhat surprising, given that many taxa experience one of their most extreme periods of predation during and immediately following metamorphosis [5–7] and that anti-predator adaptations used before or after the transition will often be unsuitable, e.g. a metamorphosing tadpole can neither swim nor hop away from a predator effectively [8]. Our lack of knowledge of transition-specific anti-predator adaptations represents a significant knowledge gap, as even a small increase in survival during this population bottleneck can have large consequences for prey population dynamics.
In this study, we investigate whether coral reef fishes possess an adaptation that reduces their loss through predation during and immediately following metamorphosis. Nearly all reef fishes have a dispersive pelagic larval phase. They arrive at the reef for the first time after hatching at or around the same time that they metamorphose into juveniles [9]. This transition is known as settlement, and during this event settling fishes choose a home site based on a number of factors, including microhabitat type [10], competitors [11] and conspecifics [10,11]. Settlers are extremely vulnerable to predation, and over 50 per cent are consumed within their first 48 h on the reef [6]. We therefore hypothesized that reef fishes preferentially settle down at predator-free sites to increase survival. Avoidance of predators at settlement is feasible because many reef-based predators are relatively sedentary [12] and patchily distributed at the scale of a few metres, leaving some patches free from key predators [13]. As the majority of settlement occurs at night when many of these predators are inactive and hiding [9], olfaction seems to be the most likely sense for their detection. Settling reef fishes have a highly developed olfactory ability [14], which they use to innately recognize and distinguish between important odours, including those from predatory and non-predatory fishes [15]. We conducted a field experiment to determine whether damselfish (Pomacentridae), a common family of reef fishes, use this olfactory ability when naturally settling to avoid reef patches manipulated to emit predator odour.
2. Material and methods
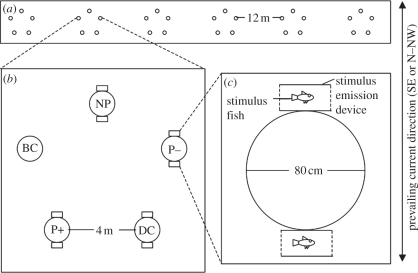
Thirty patch reefs were constructed (figure 1) on a shallow sand flat 50 m from the nearest reef. Groups of five patch reefs comprised a replicate for each day of the study. The five experimental manipulations were created using stimulus emission devices (SEDs); 3.5 l white cylindrical plastic containers with opaque mesh ends (3 mm Ø fibres surrounding 3 mm Ø holes), obscuring vision into the SED but allowing dispersion of the SED inhabitant's scent. A SED was positioned on the SE and N–NW sides of each reef, except for bare controls (BCs), with the prevailing current flowing from either of these directions. Dye tests confirmed that a patch's up-current SED dispersed scent over it. Treatment order and position were randomized among replicates and treatments were rotated within replicates daily by moving the SEDs between patches.
Figure 1.
Arrangement of experimental habitat patches at three spatial scales (top-down view; circles represent patches): (a) all six groups of five habitat patches each, (b) patch reefs within a group and manipulations assigned to them, (c) setup of a single habitat patch (50 cm high, 80% rubble and 20% live Pocillopora damicornis coral) with stimulus emission devices (SEDs).
The five treatments were: BC, which had no SEDs; SED control (DC) had empty SEDs; non-predator (NP) SEDs housed a single live Acanthurus nigrofuscus surgeonfish (a herbivore) fed ‘Hikari’ vegetarian fish food; predator without dietary damselfish chemical alarm cues (P−) SEDs housed a single live Cephalopholis microprion cod (a predator of small fish) fed squid; predator with dietary chemical alarm cues (P+) SEDs each housed a C. microprion fed commonly settling damselfish. Two predator diet treatments were used because previous studies show that chemical alarm cues from conspecifics in a predator's faeces can elicit a heightened reaction to the predator's odour [16]. Both A. nigrofuscus and C. microprion are largely inactive at night (A. L. Vail 2008, personal observation) and are therefore unlikely to have emitted significant mechanoreceptory or auditory signals, which require activity to be produced. Therefore, it is likely that odour was the only significant cue from the inhabitants of SEDs available to nocturnal settlers. All SEDs (except SED controls) contained a live stimulus fish for the duration of the study. Stimulus fish were fed daily.
The experiment ran, continuously from 28 October until 11 November 2008. Each morning (beginning at 06.30–09.00 h), all damselfish settlers on experimental patches were identified and counted by scuba divers. Damselfish, other settlers and migrants to the patches were then caught and removed.
To examine settlement choices at the scale of a single replicate (a group of five reefs on one day) and to account for spatial and temporal variation in larval settlement, settlement was converted to a replicate residual. To do so, total damselfish settlement for the replicate was divided by five, giving the number of settlers expected per reef if they were distributed evenly among treatments. A patch's residual settlement equalled its actual minus expected settlement. We determined whether residual settlement differed among treatments using ANOVAs, and for significant ANOVAs, we determined which means differed using Tukey's HSD. For the analysis of the two most commonly settling species, Pomacentrus nagasakiensis and Pomacentrus amboinensis, we only included days where the P+ stimulus cod were fed their damselfish conspecifics the previous day, allowing us to attribute differences in settlement between P− and P+ treatments to dietary conspecific cues.
3. Results
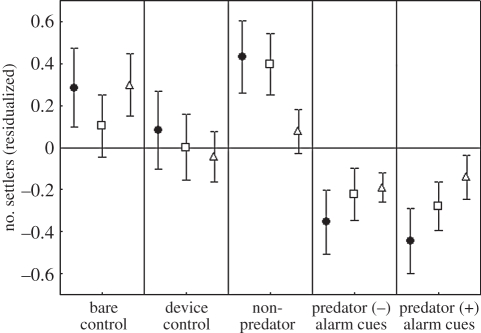
Treatment had a significant effect on settlement residualized by replicate (hereafter ‘settlers’ unless stated otherwise) for all damselfish species grouped together (F4,440 = 5.12, p < 0.0005), P. nagasakiensis (F4,340 = 3.88, p < 0.005) and P. amboinensis (F4,170 = 3.05, p < 0.02) (figure 2). For all three of these taxa, the two predator treatments had the lowest numbers of settlers. All patches emitting predator odour had fewer settlers than expected from an even settler distribution (residual settlers < 0), and all but one control had more than expected. The following significant differences were detected among treatments (Tukey's HSD <0.05): all damselfish P+ and P− < NP, P + <BC; P. nagasakiensis P+ and P− < NP; P. amboinensis P+ and P− < BC.
Figure 2.
Natural settlement choices to manipulated habitat patches made by damselfish as a family (filled circles, n = 1108) and the two most abundant damselfish species: P. nagasakiensis (squares, n = 704) and P. amboinensis (triangles, n = 109). Values displayed (mean ± s.e.) are for settlement residualized with respect to even settler distribution within the replicate.
Total settlement (summing all replicates) to predator treatments was on average 24–43% less than that to controls (table 1).
Table 1.
Total number of settlers from three damselfish taxa to manipulated habitat patches in the field. The last column gives the mean total settlement to the two predator treatments as a percentage of the mean settlement to the three controls.
| treatments without predators (no. of settlers) |
treatments with predators (no. of settlers) |
mean of treatments with predators ÷ mean of treatments without predators (as a %) | ||||
|---|---|---|---|---|---|---|
| BC | DC | NP | P− | P+ | ||
| all damselfish | 247 | 229 | 260 | 190 | 182 | 76 |
| P. nagasakiensis | 150 | 141 | 176 | 121 | 116 | 76 |
| P. amboinensis | 34 | 20 | 25 | 14 | 16 | 57 |
4. Discussion
Our study demonstrates a mechanism by which organisms undergoing a life-history transition may reduce their risk of predation during this population bottleneck. Wild reef fishes avoided habitat patches with elevated predator densities, most probably using olfaction, when naturally choosing a settlement site during their metamorphic transition from pelagic larvae to benthic juveniles. This behaviour is likely to play an important role in mitigating the extreme predation undergone by settlers.
Settlement-stage reef fishes have an acutely developed olfactory sense that they use to guide their settlement decision [14]. Our experiment provides strong evidence that settlers use olfaction to recognize and avoid predators in the wild, with all treatments emitting predator odour receiving a reduced number of damselfish settlers. To successfully avoid predators, settlers must associate predator odour with higher risk than non-predator odour. As settlement is the first post-embryonic contact these fish have with reef-based predators, their predator recognition must either be learned as embryos, learned in the pelagic environment, or be innate, as evidence from captive-reared individuals suggests [15]. Innate recognition should provide the most reliable means for predator recognition during settlement. The presence of settler conspecifics in a predator's diet had no additional impact on its avoidance by settlers. This may be owing to dietary cues being too ephemeral and unreliable in nature to inform the critical decision of settlement. In the absence of predator odour, P. nagasakiensis preferentially (although not significantly) settled to reefs experimentally manipulated to emit surgeonfish odour, suggesting that it is advantageous for P. nagasakiensis to settle where this non-predatory heterospecific is present.
Our results are likely to translate well to the reality of settlement patterns on natural patch reefs because many important reef predators are sedentary and patchily distributed at the same spatial scale as between our treatments [12], and patches free of key predators are available [13]. The absence of resident predators has been shown conclusively by predator exclusion studies to provide large survival benefits for settling and newly settled reef fishes [6]. Therefore, there is strong evidence that the predator odour avoidance by settlers demonstrated here is an anti-predator adaptation that will provide a survival benefit.
The effect of predator avoidance at settlement is likely to be complicated by non-lethal effects, which are the unavoidable costs of anti-predator responses [1], e.g. choosing a habitat because it is free from predators but sub-optimal in other respects. In aquatic systems, prey are more strongly affected by a predator's non-lethal effects than its consumptive effects [1]. By choosing a settlement site based on low predator density, reef fish will almost certainly be trading-off other important aspects of habitat quality, e.g. microhabitat type, the presence of competitors and conspecifics. It is known that these habitat factors influence prey fitness [17], often in the long term because many reef fishes are site-attached after settlement [9]. Future studies need to determine how settlers balance predator avoidance against other important habitat factors.
Many taxa undergo one of their most extreme periods of predator-induced mortality during and immediately following metamorphosis [5–7], and this study provides some of the first evidence for a strategy used to mitigate this mortality. Strong selection pressure should be placed on the evolution of such transition-specific anti-predator adaptations, making them prevalent among taxa with complex life histories. These adaptations are likely to have a significant impact on prey community dynamics through both their mitigation of predation and detrimental non-lethal effects, and thus merit further investigation.
Acknowledgements
All maintenance and experimental procedures were approved by the James Cook University Animal Ethics Committee (approval number A1067) and were in accordance with local laws.
We thank all those who assisted us in the field, the Lizard Island Research Station and its staff for infallible logistical support, and Danielle Dixson for sparking the idea for this study. Two anonymous referees provided comments that helped improve the manuscript. Funding was provided by the Australian Research Council (to M.I.M), and a Michael Hall Student Innovation Award (to A.L.V).
References
- 1.Preisser E. L., Bolnick D. I., Benard M. F. 2005. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86, 501–509 10.1890/04-0719 (doi:10.1890/04-0719) [DOI] [Google Scholar]
- 2.Bishop C. D., et al. 2006. What is metamorphosis? Integr. Comp. Biol. 46, 655–661 10.1093/icb/icl004 (doi:10.1093/icb/icl004) [DOI] [PubMed] [Google Scholar]
- 3.Johnson L. E., Strathmann R. R. 1989. Settling barnacle larvae avoid substrata previously occupied by a mobile predator. J. Exp. Mar. Biol. Ecol. 128, 87–103 10.1016/0022-0981(89)90094-4 (doi:10.1016/0022-0981(89)90094-4) [DOI] [Google Scholar]
- 4.DeVito J. 2003. Metamorphic synchrony and aggregation as antipredator responses in American toads. Oikos 103, 75–80 10.1034/j.1600-0706.2003.12527.x (doi:10.1034/j.1600-0706.2003.12527.x) [DOI] [Google Scholar]
- 5.Gosselin L. A., Qian P. 1997. Juvenile mortality in benthic marine invertebrates. Mar. Ecol. Prog. Ser. 146, 265–282 10.3354/meps146265 (doi:10.3354/meps146265) [DOI] [Google Scholar]
- 6.Almany G. R., Webster M. S. 2006. The predation gauntlet: early post-settlement mortality in reef fishes. Coral Reefs 25, 19–22 10.1007/s00338-005-0044-y (doi:10.1007/s00338-005-0044-y) [DOI] [Google Scholar]
- 7.Arnold S. J., Wassersug R. J. 1978. Differential predation on metamorphic anurans by Garter Snakes (Thamnophis): social behavior as a possible defense. Ecology 59, 1014–1022 10.2307/1938553 (doi:10.2307/1938553) [DOI] [Google Scholar]
- 8.Wassersug R. J., Sperry D. G. 1977. The relationships of locomotion to differential predation on Pseudacris triseriata (Anura: Hylidae). Ecology 58, 830–839 10.2307/1936218 (doi:10.2307/1936218) [DOI] [Google Scholar]
- 9.Leis J. M., McCormick M. I. 2002. The biology, behaviour and ecology of the pelagic, larval stage of coral reef fishes. In Coral reef fishes: dynamics and diversity in a complex system (ed. Sale P. F.), pp. 171–199 Amsterdam, The Netherlands: Academic [Google Scholar]
- 10.Ohman M. C., Munday P. L., Jones G. P., Caley M. J. 1998. Settlement strategies and distribution patterns of coral-reef fishes. J. Exp. Mar. Biol. Ecol. 225, 219–238 10.1016/S0022-0981(97)00224-4 (doi:10.1016/S0022-0981(97)00224-4) [DOI] [Google Scholar]
- 11.Sweatman H. 1988. Field evidence that settling coral reef fish larvae detect resident fishes using dissolved chemical cues. J. Exp. Mar. Biol. Ecol. 124, 163–174 10.1016/0022-0981(88)90170-0 (doi:10.1016/0022-0981(88)90170-0) [DOI] [Google Scholar]
- 12.Stewart B. D., Jones G. P. 2001. Associations between the abundance of piscivorous fishes and their prey on coral reefs: implications for prey-fish mortality. Mar. Biol. 138, 383–397 10.1007/s002270000468 (doi:10.1007/s002270000468) [DOI] [Google Scholar]
- 13.Beukers J. S., Jones G. P. 1997. Habitat complexity modifies the impact of piscivores on a coral reef fish population. Oecologia 114, 50–59 10.1007/s004420050419 (doi:10.1007/s004420050419) [DOI] [PubMed] [Google Scholar]
- 14.Atema J., Kingsford M. J., Gerlach G. 2002. Larval reef fish could use odour for detection, retention and orientation to reefs. Mar. Ecol. Prog. Ser. 241, 151–160 10.3354/meps241151 (doi:10.3354/meps241151) [DOI] [Google Scholar]
- 15.Dixson D. L., Munday P. L., Jones G. P. 2009. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 12, 1–8 10.1111/j.1461-0248.2009.01400.x (doi:10.1111/j.1461-0248.2009.01400.x) [DOI] [PubMed] [Google Scholar]
- 16.Wisenden B. D., Chivers D. P. 2006. The role of public chemical information in antipredator behaviour. In Fish communication (eds Ladich F., Collins S. P., Moller P., Kapoor B. G.), pp. 259–278 Enfield, NH: Science Publishers [Google Scholar]
- 17.Bonin M. C., Srinivasan M., Almany G. R., Jones G. P. 2009. Interactive effects of interspecific competition and microhabitat on early post-settlement survival in a coral reef fish. Coral Reefs 28, 265–274 10.1007/s00338-008-0451-y (doi:10.1007/s00338-008-0451-y) [DOI] [Google Scholar]