Abstract
Purpose
Recent advances in the treatment of ACL ruptures employ platelet-rich plasma combined with collagen to modulate growth factor release from platelets to stimulate healing. Among the most notable of these growth factors is VEGF, which is a potent mitogen and stimulator of vascular growth and healing. However, the effect of such a growth factor on healing depends on the cellular ability to bind with its receptor. The purpose of this study was to test (1) whether the strength of a tissue engineered ACL repair is associated with VEGF receptors mRNA expression of ACL cells and (2) whether age influences this association.
Methods
Nineteen female Yucatan pigs underwent enhanced ACL repair. Biomechanical testing was performed after 15 weeks of healing. Messenger RNA of VEGF receptors 1 and 2 in ACL fibroblasts as assessed by RT-PCR. The ACL structural properties were regressed on receptor expression levels in a multivariate model including serum levels of VEGF, age, and weight as potential confounders.
Results
While maximum load and linear stiffness were independent of VEGF receptor expression, VEGF receptor 1 was associated with displacement (positively) and yield load (negatively). In a multivariate model of VEGF receptor expression and biomechanics, age was associated with maximum load and yield load.
Conclusion
These findings suggest that high VEGF receptor expression, even more so at higher age, results in a more compliant scar, which in turn, may lead to greater knee laxity and a compromised clinical result.
Introduction
Tears of the anterior cruciate ligament are an important disease entity due to their high incidence and the associated risk of subsequent osteoarthritic degeneration. Recently, evidence suggests that the current gold standard of treatment for these injuries, ACL reconstruction, is also associated with an increased risk of early post-traumatic osteoarthritis in a large percentage of patients [2,4,18,18–20]. These findings have fueled new research endeavors to improve ACL treatment options by using methods of tissue engineering [40].
Tissue engineering approaches, which utilize collagenous biomaterials and platelet-rich plasma, have been used to provide a stable scaffold and growth factors to stimulate healing [3,9,10,15,33]. Among the many growth factors released from platelets, vascular endothelial growth factor (VEGF) is one of the most notable and well known for its pivotal role in wound healing [7,38]. VEGF is a growth factor that influences revascularization of healing tissues and has been shown to be associated with the strength of healing ligaments [16]. Recent evidence has shown that both VEGF and its receptors, VEGF receptor 1 and receptor 2 in particular, are expressed by ligament and tendon fibroblasts at relatively high levels [12,29,30,37] The details of VEGF signal transduction remain elusive, but it is likely that VEGF-receptor 2 is the major receptor for the growth and permeability effects of VEGF [39,1]. Receptor 1 has a more complex profile and can act as both a decoy and stimulator for receptor 2 [1,28,32,39]. Both receptors are included in this study, and it is likely that their relative interaction seen in earlier studies can also be observed in fibroblast behavior.
The study hypothesis was that VEGF receptor expression levels correlate with the structural properties of the healing ligament after ACL repair compared to untreated ACL transection. The secondary hypothesis was that the decrease in growth factor receptor density in ACL cells seen with age would also explain the decrease in healing potential of the ligament with age as previously noted in biomechanical studies [23,24,37].
Materials and Methods
Animal model
Approval for the study protocol was obtained from the Institutional Animal Care and Use Committee at the Children’s Hospital Boston, Boston, MA prior to initiating this study. A priori sample size calculations for linear regression based on estimates for the standard deviations for the values for displacement, load, and receptor expression were obtained from earlier studies [15,6,5,25,27]. Briefly, to detect an effect size of 0.75 in a regression analysis of displacement and load on VEGF receptor expression with 90% power a required sample size of sixteen was calculated according to the method described by Dupont et al [6]. Thus a sample size of 19 (16 + 20% back-up for attrition) animals was chosen. This sample size also permits detection of an effect size of 0.5 while still maintaining at least 80% power. Thus 19 female Yucatan pigs (45±21 kg of body weight) were included. These animals were chosen from a range of ages (17±16 months), to allow accounting for and assessing of the effect of age that has been described in earlier publications [23,24,37].
Collagen scaffold production
The collagen scaffolds were manufactured in our laboratory as previously described23–26. Briefly, a collagen slurry was made by solubilizing sterilely harvested, bovine connective tissue. The slurry was adjusted to a concentration of greater than 10 mg/ml. The adjusted slurry was frozen and lyophilized to create a cylindrical collagen scaffold of 30mm in length ×15 mm in diameter. All scaffolds were stored in a vacuum at −80°C until use.
PRP processing
Preoperatively, 60 cc of autologous blood was drawn into tubes containing 10% ACD (acid-citrate-dextrose, Harvest Technologies, Plymouth, MA) and spun down to isolate blood constituents. While erythrocytes were discarded, plasma and a buffy coat of platelet-rich plasma were harvested. Platelets were counted and adjusted to a five-fold concentration (5.0 ± 0.4) of the initial blood sample using the harvested plasma. The 5× platelet-concentrate was stored on ice until use.
Surgical procedure
ACL transection and ACL repair was performed as previously described [23,24,10]. All animals underwent bilateral surgery, with one ACL being transected and the contralateral ACL transected and repaired. Briefly, under general anesthesia a medial arthrotomy was made, and the fat pad was partially resected using care to protect the intermeniscal ligament. The ACL was cut at the junction of the proximal and middle third of the ligament (Figure 1a). To verify complete functional loss of the ACL, a Lachman test was performed. At this time, a small sample of ACL tissue was taken from the transection site to the laboratory for PCR analysis. The transection site was selected since it would be the site of future scar formation. This location is also the typical failure site during tensile testing. Subsequently the knee was thoroughly irrigated with 500 cc sterile saline. At this point, the knees randomly allocated for transection only were closed with out further treatment.
Figure 1.
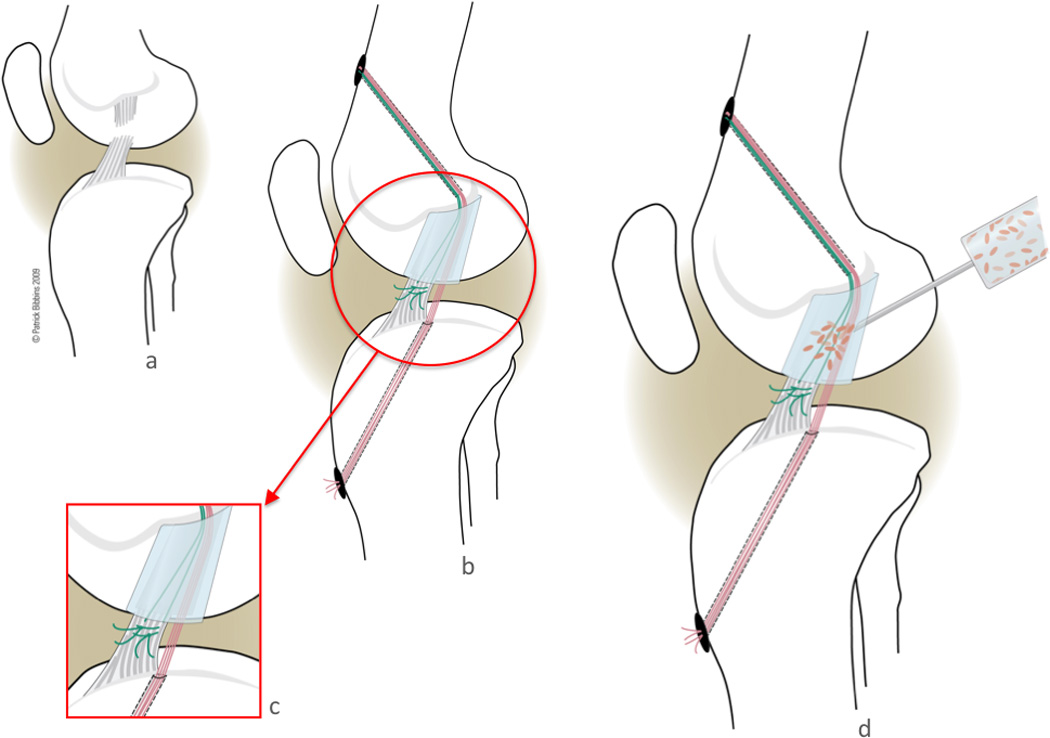
On the contralateral side, the transected ACL was repaired. To repair the transected ligament, a Kessler suture of #1 Vicryl was placed in the tibial stump exiting through the proximal cut of the ACL. An Endobutton loaded with 3 #1 Vicryl sutures (thus 6 free ends), was passed through a 4.5 mm tunnel through the femoral ACL attachment site and engaged on the lateral femoral cortex. The collagen scaffold (described above) was threaded onto 4 of the 6 free suture ends, while the remaining 2 suture limbs were reserved to tie to the ACL stump [9]. The scaffold was passed up into the femoral notch and care was taken to avoid any blood or remnants of synovial fluid soaking into the scaffold. The 4 sutures running from the femur through the scaffold were then passed through a 2.4 mm tunnel through the tibial ACL footprint. 3cc of 5× autologous PRP was placed to soak the scaffold (Figure 1d). The sutures were tensioned and tied over a button with the knee held in maximum extension (Figure 1b, c). The variable depth suture that had previously been placed in the distal ACL stump was then tied to the remaining suture limbs exiting the femoral tunnel with the knee in 30 degrees short of maximum extension to pull the tibial stump into the scaffold. The knee was closed in layers. All animals were kept under anesthesia for one hour postoperatively to allow for complete clotting and activation of the platelet concentrate. The animals were not restrained postoperatively, and were allowed ab libitum activity. At 15 weeks of healing all animals were euthanized, and the operated limb was harvested at the hip joint and stored at −20°C until mechanical testing.
Biomechanical testing
The biomechanical testing to assess the structural properties of the ligament has been previously described [8–10]. Knees were thawed to room temperature for approximately 18 hours and all extraneous muscle, the joint capsule, menisci, collateral ligaments and the PCL were dissected from the joint leaving the femur-ACL scar mass-tibia complex intact. Specimens were kept moist throughout the test protocol with a wrap of normal saline-soaked scaffolds. For failure testing, the tibia and femur were positioned so that the mechanical axis of the ACL was collinear with the load axis of the test system (MTS 810; Prairie Eden, MN). The initial knee flexion angle was set at 30°. The tibia was mounted via a sliding X-Y platform and the femur was unconstrained to axial rotation to ensure that the load was distributed evenly over the cross section of the ligament. A ramp at 20 mm/min was performed and the load-displacement data were recorded at 25Hz. From the load-displacement tracing, the displacement to yield, displacement to failure, yield load, maximum load, and linear stiffness were determined.
Biomechanical outcomes were recorded for ACL transection and ACL repair. For the purpose of statistical analyses, the difference of ACL repair over ACL transection was used in the regression model (see below).
Real time RT-PCR
All ACL biopsies obtained during surgery were washed in sterile PBS and used to establish explant cultures. The tissue was carefully examined for contamination with non-ligamentous tissue, which, if found, was generously removed. A pilot study showed insufficient mRNA yield from the biopsy itself, thus the ACL tissue was minced and placed in 100mm Petri dishes with a standard medium containing DMEM (Mediatech, Manassas, VA), 10% FBS (HyClone, South Logan, UT), 100 IU/ml Penicillin, 100 mg/ml Streptomycin, and 0.25 µg/ml Amphotericin B (Mediatech, Herndon, VA) to allow cellular outgrowth and proliferation in vitro. Medium was changed twice weekly, and tissue removed after 1 week. All cells were collected at confluence for RNA extraction.
Cells were homogenized in TRIzol® (Invitrogen), mixed with chloroform, and RNA was precipitated with isopropanol. After quantification, the RNA integrity was checked by agarose gel electrophoresis. Oligotex® (Qiagen), mRNA purification reagent, was used to purify mRNA from total RNA according to standard protocols. RETROscript™ (Ambion), first strand synthesis kit, was used to reverse transcribe the mRNA to cDNA. In brief, mRNA (100 ng) was reverse transcribed with M-MLV reverse transcriptase in 20 µl. The cDNA was diluted 5× and used as template for RT-PCR.
Real-time quantitative RT-PCR was used to evaluate mRNA levels of VEGF receptor 1 (VEGF-R1) and receptor two (VEGF-R2) using an ABI PRISM® 7700 sequence detection system (Applied Biosystems). The real-time RT-PCR conditions were described previously [11,13]. Briefly, the reactions were run in triplicate, each containing 1 µl cDNA along with 2.5 pmoles of gene specific primers (Table 1) in a final volume of 25 µl with the following thermal profile: 5 minutes initial denaturing step to activate the DNA polymerase, then 40 cycles each at 95°C for 35 sec, 60°C for 45 sec, and 72°C for 45 sec. Levels of GAPDH transcript proved to be stable under the experimental trials; therefore it was selected as a reference gene. PCR was done in triplicates for each sample.
Table 1.
Primers sequences used for PCR
| target | Forward | Backward |
|---|---|---|
| VEGR-R1 | agc cca agg cct cac tca ag | acg gga ggg ctg cac tac ag; |
| VEGR-R2 | ctg gtt ctg gcc caa caa tc | tgg tcc cca gac atg gaa tc |
| GAPDH | aag ggc atc ctg ggc tac ac | ggt cca ggg gct ctt act cc |
Serum VEGF levels
Pre-operatively blood was drawn through a large bore needle (18g or larger) into serum separator tubes and allowed to clot overnight. Aliquots of porcine serum were collected and stored at −80°C until further processing. Serum levels of VEGF were measured using commercially available ELISA kits (VEGF Quantikine ELISA Kit, Minneapolis, MN, USA) according to the manufacturer’s protocol. VEGF levels were measured in triplicates for each sample.
Statistical Analysis
All outcomes were measured with at least 3 decimals measurement accuracy. Continuous variables were tested for normality using the Shapiro-Wilk test and normal probability plots and were found to have a normal distribution. Subsequently, a multilevel mixed-effects regression model to test for associations between receptor expression and biomechanics was created using treatment (ACL transection versus ACL repair) as within subject variable. Such a model regresses the relative effect of ACL repair over ACL transection on receptor expression, i.e. telling how much ACL repair improves functional outcome over a torn ACL, while accounting for the effect of covariates. Age, body weight, and serum VEGF levels were included as potential confounders [34,37]. A confounder is a third variable, that affects (confounds) or even causes an observed association between two variables. In this case, a found association between VEGF receptors and biomechanics might be spurious, because it is really age that causes lower VEGF receptor levels and poor biomechanics without an actual, direct association between those two parameters. Multivariate regression allows testing for the presence and estimating the size of such an effect. Age was used as a linear variable, as well as an ordinal variable with three groups: immature, adolescent, adult. In consistency with earlier studies, these groups were based on physeal closure, with open physes for immature animals, closed of adult, and closing but not fully closed for adolescent animals. The regression model was build in a backward stepwise manner, i.e. all variables were included initially and those variables with no significant association were excluded one by one while the model was re-run after each exclusion until no further improvement in model fit (using likelihood ratio tests) over the previous model was seen. All analyses were done using intercooled STATA 10 (Stata Corp LP, College Station, TX). An alpha value of 5% was considered significant. All results are given as mean ± SD with 95% confidence intervals [35].
Results
Surgery & Animal welfare
In the peripheral blood, there were no preoperative differences in red blood cell (p=n.s.), white blood cell (p=n.s.), or platelet counts (p=n.s.) between the three age groups ruling out that difference in PRP constituents as causes for biomechanical differences. All animals tolerated the surgery well and reached full weight bearing status with 24 hours post-op. There were no postoperative complications and all animals reached the analysis stage in good health.
Displacement
Displacement to yield and failure showed significant associations with VEGF-R1, but not with VEGF-R2. (Table 2) In the multivariate model, there was no evidence that the effect of VEGF-R1 or R2 was confounded by animal age, or the other covariates weight, or serum VEGF levels. (Tables 3 and 4) After adjusting for weight, the association between displacement to yield and VEGFR2 was substantially strengthened and was borderline significant (p=0.081). (Table 4)
Table 2.
Coefficients from univariate regression for VEGF-R1 & R2
| Outcome | Variable | coef | 95% CI | p-value | |
|---|---|---|---|---|---|
| LL | UL | ||||
| Displacement to Yield (mm) | VEGF-R1 | 0.6 | 0.3 | 0.9 | <0.01 |
| VEGF-R2 | 0.1 | −0.0 | 0.3 | n.s. | |
| Displacement to Failure (mm) | VEGF-R1 | 0.6 | 0.3 | 0.9 | <0.01 |
| VEGF-R2 | 0.1 | −0.1 | 0.3 | n.s. | |
| Yield Load (N) | VEGF-R1 | 0.8 | −9.4 | 11.0 | n.s. |
| VEGF-R2 | 1.2 | −4.3 | 6.6 | n.s. | |
| Maximum Load (N) | VEGF-R1 | 2.8 | −7.3 | 12.9 | n.s. |
| VEGF-R2 | 0.9 | −4.6 | 6.4 | n.s. | |
| Stiffness (N/mm) | VEGF-R1 | 0.2 | −1.6 | 1.9 | n.s. |
| VEGF-R2 | 0.0 | −0.9 | 1.0 | n.s. | |
Coef=regression coefficient, 95%CI=95% confidence interval, UL=upper level, LL=lower level
Table 3.
Coefficients from the full regression models for VEGF-R1
| Outcome | Variable | coef | 95% CI | p-value | |
|---|---|---|---|---|---|
| LL | UL | ||||
| Displacement to Yield (mm) | VEGF-R1 | 0.6 | 0.3 | 0.9 | <0.01 |
| Weight | 0.1 | 0.0 | 0.1 | n.s. | |
| Age | −1.2 | −3.7 | 1.3 | n.s. | |
| Serum VEGF | 0.0 | −0.1 | 0.2 | n.s. | |
| Displacement to Failure (mm) | VEGF-R1 | 0.6 | 0.3 | 0.9 | <0.01 |
| Weight | 0.0 | 0.0 | 0.1 | n.s. | |
| Age | −1.2 | −3.9 | 1.4 | n.s. | |
| Serum VEGF | 0.0 | −0.2 | 0.2 | n.s. | |
| Yield Load (N) | VEGF-R1 | −1.0 | −10.8 | 8.8 | n.s. |
| Weight | 1.1 | −1.1 | 3.3 | n.s. | |
| Age | −95.3 | −181.0 | −9.7 | 0.03 | |
| Serum VEGF | −4.1 | −10.1 | 2.0 | n.s. | |
| Maximum Load (N) | VEGF-R1 | 1.1 | −8.6 | 10.8 | n.s. |
| Weight | 0.7 | −1.4 | 2.9 | n.s. | |
| Age | −87.3 | −171.6 | −3.0 | 0.04 | |
| Serum VEGF | −4.9 | −10.9 | 1.0 | n.s. | |
| Stiffness (N/mm) | VEGF-R1 | 0.1 | −1.6 | 1.7 | n.s. |
| Weight | −0.2 | −0.6 | 0.2 | n.s. | |
| Age | −4.0 | −18.3 | 10.4 | n.s. | |
| Serum VEGF | −1.1 | −2.1 | −0.1 | 0.03 | |
Coef=regression coefficient, 95%CI=95% confidence interval, UL=upper level, LL=lower level
Table 4.
Coefficients from the full regression models for VEGF-R2
| Outcome | Variable | coef | 95% CI | p-value | |
|---|---|---|---|---|---|
| LL | UL | ||||
| Displacement to Yield (mm) | VEGF-R2 | 0.2 | 0.0 | 0.3 | 0.08 |
| Weight | 0.1 | 0.0 | 0.1 | 0.05 | |
| Age | −1.9 | −4.5 | 0.7 | n.s. | |
| Serum VEGF | 0.0 | −0.1 | 0.2 | n.s. | |
| Displacement to Failure (mm) | VEGF-R2 | 0.1 | 0.0 | 0.3 | n.s. |
| Weight | 0.6 | 0.0 | 0.1 | n.s. | |
| Age | −2.0 | −4.7 | 0.8 | n.s. | |
| Serum VEGF | 0.0 | −0.2 | 0.2 | n.s. | |
| Yield Load (N) | VEGF-R2 | −0.03 | −5.3 | 5.3 | n.s. |
| Weight | 1.0 | −1.1 | 3.2 | n.s. | |
| Age | −94.1 | −178.9 | −9.3 | 0.03 | |
| Serum VEGF | −4.1 | −10.1 | 1.9 | n.s. | |
| Maximum Load (N) | VEGF-R2 | −0.5 | −5.7 | 4.8 | n.s. |
| Weight | 0.7 | −1.4 | 2.9 | n.s. | |
| Age | −88.8 | −172.3 | −5.3 | 0.04 | |
| Serum VEGF | −4.9 | −10.9 | 1.0 | n.s. | |
| Stiffness (N/mm) | VEGF-R2 | −0.2 | −1.1 | 0.6 | n.s. |
| Weight | −0.2 | −0.5 | 0.2 | n.s. | |
| Age | −4.1 | −18.3 | 10.1 | n.s. | |
| Serum VEGF | −0.2 | −0.5 | 0.2 | n.s. | |
Coef=regression coefficient, 95%CI=95% confidence interval, UL=upper level, LL=lower level, * represents a mulitvariate regression adjusting for confounders.
Load
There was no evidence for an association between maximum or yield load and VEGF-R1 or R2 expression. (table 2) In the multivariate model, there were significant associations between age and maximum and yield load for both receptors suggesting that age accounts for part of the effect of VEGF receptors on biomechanical outcomes. (Tables 3 and 4).
Stiffness
There were no associations of stiffness with VEGF-R1 expression or VEGF-R2 expression. (Table 2) The multivariate model showed no associations with age. (Tables 3 and 4)
Discussion
The most important finding of the present study was strong evidence for an association displacement to yield and failure after enhanced ACL repair with VEGF receptor expression. No evidence was found for a direct influence of VEGF receptor density on the maximum load for the healing ligaments. Finally, strong evidence emerged that the association between VEGF receptor expression and load was affected by age, suggesting that age masks at least some of the association between these two factors. This interpretation is supported by findings from prior studies [23,24,37].
VEGF is a well-known factor in tissue healing, which is primarily associated with endothelial cells and angiogenesis. Recently, the role of VEGF in ligament and tendon healing has been studied more closely. Such analyses showed that fibroblasts, especially during wound healing, release VEGF [11,12,29,30] and express VEGF-receptors [12,37], both in vitro and in a graft used for ACL reconstruction [30]. Other recent evidence shows that VEGF addition to grafts for ACL reconstruction results in increased AP laxity and reduced linear stiffness [40]. However, the exact function of VEGF and, especially, VEGF-receptors in fibroblasts, is still elusive.
This study showed significant associations between displacement and VEGF-receptor 1 expression, but none with VEGF-receptor 2. A greater expression of VEGF-receptor at the time of surgery resulted in greater displacements to yield and failure. The finding that this association exists only for receptor 1 may be due to the fact that the activity of receptor 2, which is the main receptor in VEGF transduction, is modified by receptor 1 [31]. These findings suggest that a higher VEGF receptor expression results in a more extensible repair tissue.
There was no association of load, either a t yield or at maximum, with either VEGF receptor expression. More interestingly significant associations were seen between age and load, which were slightly attenuated after adjusting for receptor expression levels. These findings suggest that VEGF receptors play only a small role in the association between age and load that has been described earlier [23,24]. Load is a function of fibroblastic synthetic activity, which has been shown to be enhanced by PRP [3]. The independence of load from VEGF receptors, despite the proven stimulation of fibroblast bioactivity by PRP [23,24,3], might be interpreted as an indicator of the higher importance of other PRP-related growth factors, such as TGF-beta or PDGF [7,38]. Such an interpretation is in accordance with findings from earlier studies, showing that TGF-beta might counteract any VEGF-mediated detrimental action in scar formation [14].
The third studied parameter was linear stiffness. Stiffness, as a compound parameter of load over displacement, and thus offers a more comprehensive evaluation of ACL healing. As seen in earlier studies, there was a significant, negative association between serum VEGF levels and stiffness [40,30]. However, there was no association with VEGF receptors. Interestingly, there was, again, strong evidence for residual confounding by age.
Also, strong evidence was found that the association between yield load and maximum load and VEGF receptor expression was influenced by age, in such a way as that accounting for age reduced the strength of the association between load and VEGF receptors. Recent studies have shown repeatedly and consistently that age is negatively associated with functional outcome after PRP-augmented primary ACL repair, as well as tissue maturation and ACL fibroblast migration, proliferation and collagen production [23–25] suggesting growth factor receptor expression as a reason for the observed age-dependence.
These findings have potential for important clinical implications. Since they describe the interaction of two important determinants of functional outcome of enhanced ACL repair, the identification of these determinants allows using a tailored therapeutic approach based on individual patient characteristics. Second, a number of drugs have been developed that influence the VEGF pathway and might be added to such a surgical technique. Finally, these findings suggest the overstimulation of VEGF receptors might lead to a poorer functional result, implying that higher concentrations of platelets in PRP might not necessarily produce better results in sports medicine [22].
This study has potential limitations. Only one growth factor was studied, thus potential interaction between different growth factors could not be assessed. Secondly, the data build on a snapshot of receptor expression, which might change in response to PRP. Lastly, no post-translational control on the peptide level to confirm the mRNA changes, but earlier research confirmed PRP-initiated up-regulation of VEGFR-1 and R2 mRNA expression by histology and immunohistochemistry [17].
Conclusion
In conclusion, the findings of this study suggest that VEGF receptor expression in ACL fibroblasts is associated displacement, but not load or stiffness, after ACL repair compared to untreated ACL transection. High VEGF receptor expression is associated with higher displacement, suggesting a more plastic repair tissue. Also, the age-dependent expression of VEGF receptors seems to be an important factor in the age-dependence of functional outcome after ACL repair. Most likely this is also true for other growth factors. This interpretation suggests that the bottleneck of cellular stimulation is reception, not concentration of growth factors. Consequently, increasing PRP concentrations will not improve outcome but might very well increase the risk of adverse events [22]. Addressing growth factor reception might prove to be a more effective treatment modification.
Acknowledgement
This study was funded by the NIH NIAMS grant R01 AR052772 and NIH Grant AR054099.
Footnotes
The senior author is a founder and stockholder in Connective Orthopaedics, the first author is a consultant for Connective Orthopaedics. Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Casteleyn P. Management of anterior cruciate ligament lesions: surgical fashion, personal whim or scientific evidence? Study of medium and long-term results. Acta Orthop Belg. 1999;65:327–339. [PubMed] [Google Scholar]
- 3.Cheng M, Wang H, Yoshida R, Murray MM. Platelets and Plasma Proteins Are Both Required to Stimulate Collagen Gene Expression by Anterior Cruciate Ligament Cells in Three-Dimensional Culture. Tissue Eng Part A. 2009;16(5):1479–1489. doi: 10.1089/ten.tea.2009.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen M, Amaro JT, Ejnisman B, Carvalho RT, Nakano KK, Peccin MS, Teixeira R, Laurino CF, Abdalla RJ. Anterior cruciate ligament reconstruction after 10 to 15 years: association between meniscectomy and osteoarthrosis. Arthroscopy. 2007;23(6):629–634. doi: 10.1016/j.arthro.2007.03.094. [DOI] [PubMed] [Google Scholar]
- 5.Dupont WD, Plummer WD. Power and sample size calculations: a review and computer program. Control Clin Trial. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 6.Dupont WD, Plummer WD., Jr Power and Sample Size Calculations for Studies Involving Linear Regression. Controlled Clinical Trials. 1998;19(6):589. doi: 10.1016/s0197-2456(98)00037-3. [DOI] [PubMed] [Google Scholar]
- 7.Everts PA, Knape JT, Weibrich G, Schonberger JP, Hoffmann J, Overdevest EP, Box HA, van Zundert A. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174–187. [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26(11):1500–1505. doi: 10.1002/jor.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming BC, Magarian EM, Harrison SL, Paller DJ, Murray MM. Collagen scaffold supplementation does not improve the functional properties of the repaired anterior cruciate ligament. J Orthop Res. 2010;28(6):703–709. doi: 10.1002/jor.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming BC, Spindler KP, Palmer M, Magarian E, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing ACL grafts in a porcine model. Am J Sports Med. 2009;37(8):1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofstaetter JG, Saad FA, Samuel RE, Wunderlich L, Choi YH, Glimcher MJ. Differential expression of VEGF isoforms and receptors in knee joint menisci under systemic hypoxia. Biochem Biophys Res Commun. 2004;324(2):667–672. doi: 10.1016/j.bbrc.2004.09.103. [DOI] [PubMed] [Google Scholar]
- 12.Hofstaetter JG, Saad FA, Sunk IG, Bobacz K, Friehs I, Glimcher MJ. Age-dependent expression of VEGF isoforms and receptors in the rabbit anterior cruciate ligament. Biochim Biophys Acta. 2007;1770(7):997–1002. doi: 10.1016/j.bbagen.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Hofstaetter JG, Wunderlich L, Samuel RE, Saad FA, Choi YH, Glimcher MJ. Systemic hypoxia alters gene expression levels of structural proteins and growth factors in knee joint cartilage. Biochem Biophys Res Commun. 2005;330(2):386–394. doi: 10.1016/j.bbrc.2005.02.168. [DOI] [PubMed] [Google Scholar]
- 14.Hou Y, Mao Z, Wei X, Lin L, Chen L, Wang H, Fu X, Zhang J, Yu C. Effects of transforming growth factor-beta1 and vascular endothelial growth factor 165 gene transfer on Achilles tendon healing. Matrix Biol. 2009;28(6):324–335. doi: 10.1016/j.matbio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37(12):2401–2410. doi: 10.1177/0363546509339915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kessler MA, Behrend H, Henz S, Stutz G, Rukavina A, Kuster MS. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- 17.Kilian O, Alt V, Heiss C, Jonuleit T, Dingeldein E, Flesch I, Fidorra U, Wenisch S, Schnettler R. New blood vessel formation and expression of VEGF receptors after implantation of platelet growth factor-enriched biodegradable nanocrystalline hydroxyapatite. Growth Factors. 2005;23(2):125–133. doi: 10.1080/08977190500126306. [DOI] [PubMed] [Google Scholar]
- 18.Lind M, Menhert F, Pedersen AB. The first results from the Danish ACL reconstruction registry: epidemiologic and 2 year follow-up results from 5,818 knee ligament reconstructions. Knee Surg Sports Traumatol Arthrosc. 2008 doi: 10.1007/s00167-008-0654-3. [DOI] [PubMed] [Google Scholar]
- 19.Lohmander LS, Englund M, Dahl L, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 20.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 21.Mastrangelo A, Magarian E, Palmer M, Vavken P, Murray M. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2009;28(5):644–651. doi: 10.1002/jor.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastrangelo A, Vavken P, Fleming B, Harrison S, Murray M. Reduced platelet concentration does not harm PRP effectiveness for ACL repair in a porcine in vivo model. J Orthop Res. 2011 doi: 10.1002/jor.21375. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mastrangelo AN, Haus BM, Vavken P, Palmer MP, Machan JT, Murray MM. Immature animals have higher cellular density in the healing anterior cruciate ligament than adolescent or adult animals. J Orthop Res. 2010;28(8):1100–1106. doi: 10.1002/jor.21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastrangelo AN, Magarian EM, Palmer MP, Vavken P, Murray MM. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2010;28(5):644–651. doi: 10.1002/jor.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray MM, Magarian EM, Harrison SL, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039–2049. doi: 10.2106/JBJS.I.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray MM, Martin SD, Martin TL, Spector M. Histological Changes in the Human Anterior Cruciate Ligament After Rupture. J Bone Joint Surg Am. 2000;82(10):1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res. 2009;27(5):639–645. doi: 10.1002/jor.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odorisio T, Schietroma C, Zaccaria M, Cianfarani F, Tiveron C, Tatangelo L, Failla C, Zambruno G. Mice overexpressing placenta growth factor exhibit increased vascularization and vessel permeability. J Cell Sci. 2002;115:2559–2567. doi: 10.1242/jcs.115.12.2559. [DOI] [PubMed] [Google Scholar]
- 29.Petersen W, Pufe T, Unterhauser F, Zantop T, Mentlein R, Weiler A. The splice variants 120 and 164 of the angiogenic peptide vascular endothelial cell growth factor (VEGF) are expressed during Achilles tendon healing. Arch Orthop Trauma Surg. 2003;123(9):475–480. doi: 10.1007/s00402-003-0490-3. [DOI] [PubMed] [Google Scholar]
- 30.Petersen W, Unterhauser F, Pufe T, Zantop T, Sudkamp NP, Weiler A. The angiogenic peptide vascular endothelial growth factor (VEGF) is expressed during the remodeling of free tendon grafts in sheep. Arch Orthop Trauma Surg. 2003;123(4):168–174. doi: 10.1007/s00402-002-0462-z. [DOI] [PubMed] [Google Scholar]
- 31.Petrova T, Makinen T, Alitalo K. Signaling via vascular endothelial growth factor receptors. Experimental Cell Research. 1999;253(117) doi: 10.1006/excr.1999.4707. [DOI] [PubMed] [Google Scholar]
- 32.Rahimi N. VEGFR-1 and VEGFR-2: two non-identical twins with a uniqu physiognomy. Front Biosci. 2006;11:818–829. doi: 10.2741/1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spindler KP, Murray MM, Carey JL, Zurakowski D, Fleming BC. The use of platelets to affect functional healing of an anterior cruciate ligament (ACL) autograft in a caprine ACL reconstruction model. J Orthop Res. 2009;27(5):631–638. doi: 10.1002/jor.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vavken P, Culen G, Dorotka R. Management of confounding in controlled orthopaedic trials: a cross-sectional study. Clin Orthop Relat Res. 2008;466(4):985–989. doi: 10.1007/s11999-007-0098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vavken P, Heinrich KM, Koppelhuber C, Rois S, Dorotka R. The Use of Confidence Intervals in Reporting Orthopaedic Research Findings. Clin Orthop Relat Res. 2009;467(12):3334–3339. doi: 10.1007/s11999-009-0817-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vavken P, Murray MM. Translational Studies in ACL repair. Tissue Eng Part B. 2009;16(1):5–11. doi: 10.1089/ten.teb.2009.0147. [DOI] [PubMed] [Google Scholar]
- 37.Vavken P, Saad F, Murray M. Age-dependence of expression of growth factor receptors in porcine ACL fibroblasts. J Orthop Res. 2010;28(8):1107–1112. doi: 10.1002/jor.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 39.Yancopoulos G, Davis S, Gale N, Rudge J, Wiegand S, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 40.Yoshikawa T, Tohyama H, Katsura T, Kondo E, Kotani Y, Matsumoto H, Toyama Y, Yasuda K. Effects of local administration of vascular endothelial growth factor on mechanical characteristics of the semitendinosus tendon graft after anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2006;34(12):1918–1925. doi: 10.1177/0363546506294469. [DOI] [PubMed] [Google Scholar]


