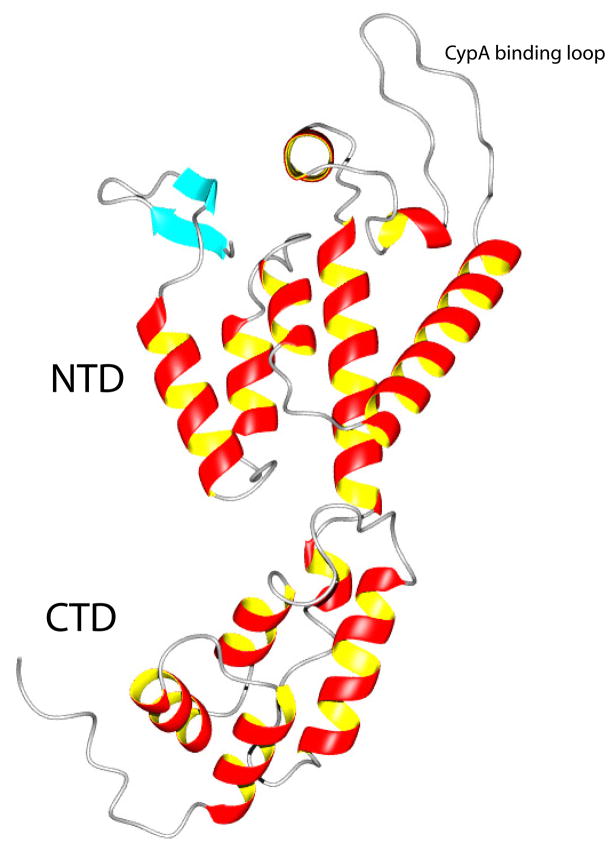
Figure 4.
A representative member of the NMR structures of the full-length monomeric mutant CA as a ribbon diagram (MolMol). The secondary structure consists of a short β-hairpin (aqua blue color), with helices 1 to 7 in the NTD domain at the top and helices 8 to 12 (including the short 310 - helix as helix 8) in the CTD domain at the bottom. Even though our construct has the start codon residue Met (unassigned) prior to Pro1, the general arrangement of the β-hairpin is very similar to that seen in the isolated NTD structure (16). The linker between the NTD and CTD domains is five residues long (residues 145 to 149 corresponding to YSPTS) and highly flexible in solution (see Figure 5). In the crystal structure of the antiparallel dimer of wt CA, it is two residues long (SP) (24). The cyclophilin A (CypA) binding loop (16) is located between helices 4 and 5 at upper right.