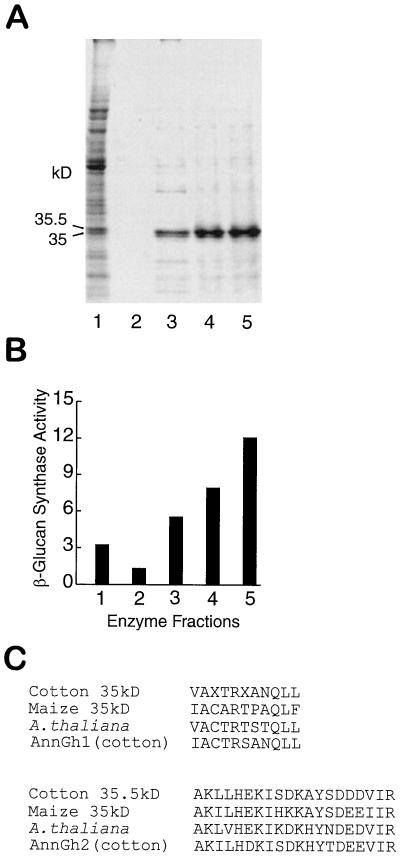
Figure 1.
Copurification of annexins with β-glucan synthase from cotton fibers. A, Protein profiles of different enzyme fractions on an SDS-PAGE gel stained with Coomassie blue. Lane 1, SE1, 15 μg/lane. Lanes 2, 3, 4, and 5, Proteins released from product entrapment of the SE1 fraction that was performed with different Ca2+ concentrations of 0, 1, 2, and 4 mm, respectively. B, β-Glucan synthase assay for the fractions in A. β-Glucan synthase activity is represented as units, where 1 unit = 1 nmol Glc incorporated min−1. C, Partial amino acid sequences obtained from the 35- and 35.5-kD bands are aligned with homologous regions in annexins from maize (35 kD; Battey et al., 1996), Arabidopsis (A. thaliana; Gidrol et al., 1996), and cotton fibers (AnnGh1 and AnnGh2; Delmer and Potikha, 1997).