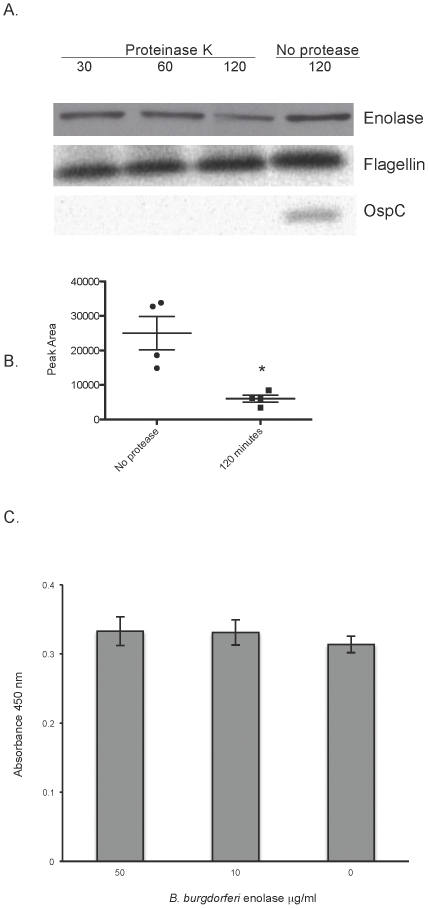
Figure 2. B. burgdorferi enolase is surface exposed.
(A) Demonstration of outer surface exposure of enolase by in situ protease degradation. Whole B. burgdorferi were incubated with proteinase K for 30, 60 or 120 minutes or 120 minutes in buffer with no protease. Proteases were then inactivated, bacteria were lysed, proteins were separated by SDS-PAGE, and the integrity of enolase, OspC (outer surface protein), and FlaB (periplasmic protein) were analyzed by immunoblot. Two separate protease degradation experiments were performed, with blots repeated 4 times. A representative blot is shown. (B) Densitometric analysis of in situ protease degradation immunoblots. Images from blots were scanned and peak areas for 120-minute proteinase K treatment and no protease control were assessed using ImageJ software (http://imageJ.nih.gov/ij). Data represent the means and standard errors from 4 separate blots. *, P = 0.03, Student's t test assuming unequal variances. (C) Binding of exogenous B. burgdorferi enolase to the surface of whole bacteria. Binding of B. burgdorferi enolase (0–50 µg/ml) to immobilized B. burgdorferi (2×107/ml) was analyzed by ELISA, with bound enolase detected by rabbit polyclonal antiserum directed against human enolase. Data represent the means and standard errors from three separate experiments with six replicates per enolase concentration.