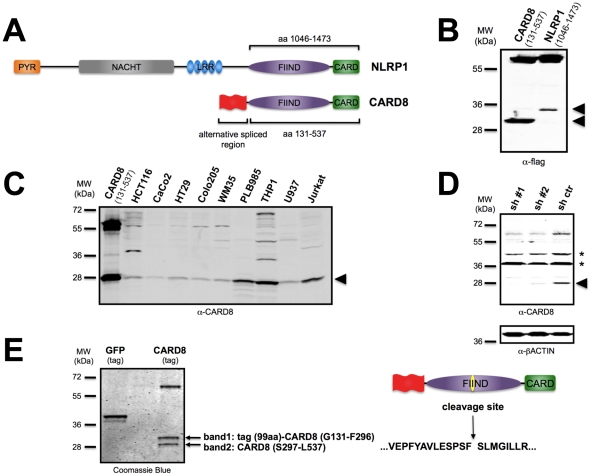
Figure 1. CARD8 and NLRP1 undergo cleavage within the FIIND.
(A) Schematic representation of NLRP1 and CARD8 domains structure. The CARD8 region for which numerous isoforms arise through alternative mRNA splicing is indicated in red. The regions of the CARD8 and NLRP1 proteins that contain the FIIND and CARD and that were expressed in cells are shown. (B) HEK293T cells were transiently transfected with plasmids encoding the FIIND-CARD region of CARD8 (left) or NLRP1 (right) with N-terminal flag tags. After 24 hours, cell lysates were prepared and analyzed by SDS-PAGE/immunoblotting using anti-flag antibody. Unexpected smaller forms of the flag-tagged proteins are indicated by arrow heads. Molecular weight markers are indicated in kilo-Daltons (kDa). (C) Analysis of endogenous CARD8. Cell lysates from the indicated cancer and leukemia cell lines were normalized for total protein content and subjected to SDS-PAGE/immunoblot analysis using rabbit antiserum directed against the C-terminal portion of CARD8. The smaller form of CARD8 is indicated by arrowhead. (D) HCT116 cells were stably transduced with lentiviruses encoding either two different shRNAs or scrambled control. Cell lysates were analyzed by immunoblotting using a rabbit anti-CARD8 antibody. Full-length CARD8 migrates at molecular mass of ∼60 kD, whereas cleaved CARD8 appears at ∼28 kD (arrowhead). As a loading control, membranes were re-probed with anti-ß-actin antibody. Asterisks indicate non-specific bands. (E) HEK293T cells stably expressing SBP-tagged proteins were lysed and incubated with magnetic streptavidin beads, followed by elution with biotin. Eluted proteins were subjected to SDS-PAGE and stained with Comassie Blue. The bands indicated by an arrow (band 1; band 2) were subjected to tryptic digestion and analyzed by LC-MS to reveal peptides corresponding to a novel cleavage site between Phe-296 and Ser-297.