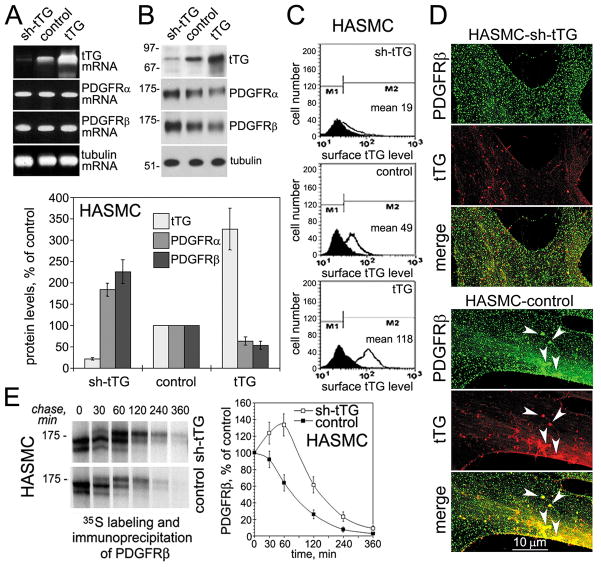
Figure 1. tTG regulates PDGFRβ receptor levels and localization in vascular SMCs.
(A) tTG does not alter PDGFRα and PDGFRβ mRNA levels in vascular SMCs. mRNA levels of tTG, PDGFRα, PDGFRβ, and tubulin were determined by quantitative RT-PCR in human aortic SMCs expressing sh-tTG, control vector, and tTG. (B) tTG down-regulates PDGFRα and PDGFRβ levels in vascular SMCs. The levels of tTG, PDGFRα, PDGFRβ, and tubulin were defined by immunoblotting. The amounts of tTG, PDGFRα, and PDGFRβ were quantified by densitometry, averaged, and expressed as % change compared to those in control cells. (C) Surface tTG levels were defined by immunostaining of live nonpermeabilized cells and flow cytometry. (D) tTG promotes PDGFRβ clustering in vascular SMCs. Human aortic SMCs that express non-silensing control (HASMC-control) or tTG (HASMC-shtTG) shRNA were double stained for PDGFRβ (green) and tTG (red). Note large PDGFRβ clusters containing tTG in the lamellae of HASMSC-control cells (arrowheads, bottom) and their lack in tTG-deficient HASMC-shtTG cells (top). (E) tTG promotes PDGFRβ turnover. HASMC-shtTG and HASMC-control cells were pulse-labeled with Tran35S-label™ for 30 min without PDGF-BB and then chased for indicated times in the absence of growth factors. PDGFRβ was immunoprecipitated from SDS-denatured cell extracts containing 200 μg total cell protein. The immune complexes were resolved by SDS-PAGE and detected by fluorography. The amounts of de novo synthesized PDGFRβ were defined by 35S scintillation counting and presented as percentages of those in the cells before start of the chase. (B,E) Shown are the means ± S.D. for three independent experiments.