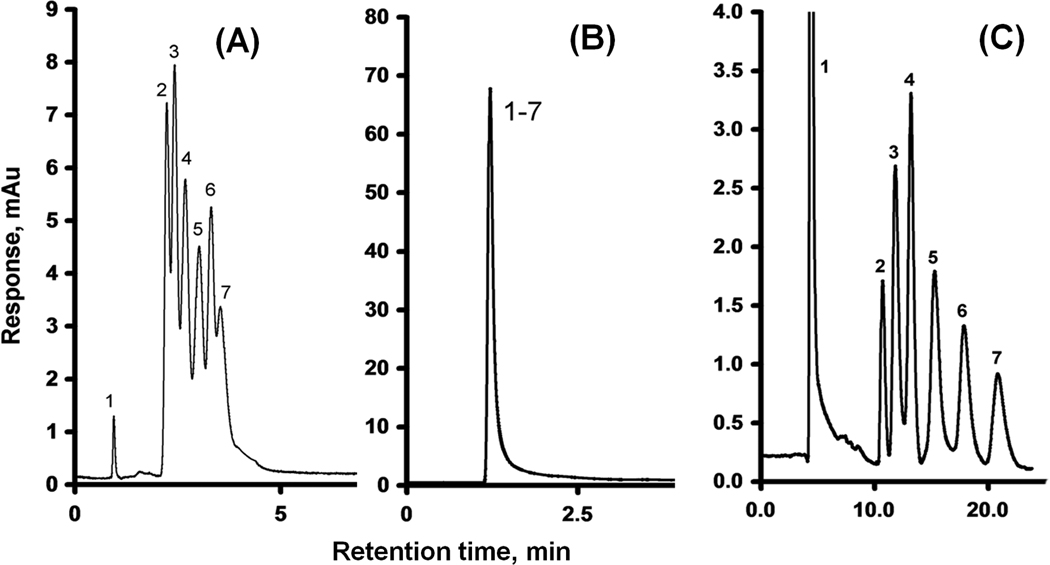
Fig. 19.
Separation of uracil and alkylbenzenes using a monolithic poly(glycidyl methacrylate-co-ethylene dimethacrylate) capillary column (A), the previous column reacted with ammonia (B), and column modified with oxidized carbon nanotubes (C). Conditions: (A) Column, 180 mm × 100 µm i.d., mobile phase 45% acetonitrile-5% THF-50% water, flow rate 1.00 µL/min, back pressure 16 MPa; (B) Column, 200 mm × 100 µm i.d., mobile phase 50% acetonitrile-50% water, flow rate 1.00 µL/min, back pressure 17 MPa; (C) Column, 170 mm × 100 µm i.d., mobile phase 47.5% acetonitrile-2.5% THF-50% water- mixture; flow rate 0.25 µL/min, back pressure 30 MPa; Peaks: uracil (1), benzene (2), toluene (3), ethylbenzene (4), propylbenzene (5), butylbenzene (6), and amylbenzene (7). Adapted from ref. [94].