Abstract
We examined biomarkers of selenium status (whole blood Se, serum Se, and glutathione peroxidase activity) and thyroid status (concentrations and ratios of thyroxine, T4, and tri-iodothyronine, T3, and albumin) in polar bears to assess variations among cohorts, and relationships to circulating concentrations of contaminants. Concentrations of total mercury (Hg) in whole blood were similar among cohorts (prime aged males and females, older animals ages ≥ 16 years, and young animals ages 1 – 5 years; 48.44 ± 35. 81; p = 0.253). Concentrations of sum of seven polychlorinated biphenyls (ΣPCB7) in whole blood were greater in females (with and without cubs, 26.44 ± 25.82 ng/g ww) and young (26.81 ± 10.67 ng/g ww) compared to males (8.88 ± 5.76 ng/g ww, p < 0.001), and significantly related to reduced body condition scores (p < 0.001). Concentrations of Se and albumin were significantly greater in males than females (whole blood Se, males, 42.34 pmol/g ww; females, 36.25 ± 6.27 pmol/g ww, p = 0.019; albumin, males, 4.34 ± 0.34 g/dl, females, 4.10 ± 0.29 g/dL, p = 0.018). Glutathione peroxidase activity ranged from 109.1 – 207.8 mU/mg hemoglobin, but did not differ significantly by sex or age (p > 0.08). Thyroid hormones were greater in females (solitary females and females with cubs) compared to males (p < 0.001). Biomarkers of Se status and concentrations of T3 were significantly positively related to Hg in all prime aged polar bears (p < 0.03). Albumin concentrations were significantly positively related to total TT4, and significantly negatively related to concentrations of ΣPCB7 (p < 0.003). Total thyroxine (TT4) was significantly negatively associated with blood concentrations of ΣPCB7 in solitary females (p = 0.045). These data suggest that female polar bears were more susceptible to changes in blood-based biomarkers of selenium and thyroid status than males. Further classifications of the physiologic states of polar bears and repeated measures of individuals over time are needed to accurately assess the biological impact of combined toxicant exposures.
Keywords: Body condition, Heavy metals, Lipophilic contaminants, Thyroid binding proteins, Whole blood
1. Introduction
Polar bears (Ursus maritimus) are apex predators of the arctic marine ecosystem and exposed to a combination of inorganic and organic toxicants that bioaccumulate and biomagnify (Atwell et al. 1998; Bentzen et al. 2008; Cardona-Marek et al. 2009; Kucklick et al. 2002). Climate changes may negatively impact the health of polar bears and other arctic marine mammals by altering the transmission of disease agents and exposure to contaminants (Burek et al. 2008; Jenssen 2006; Letcher et al. 2009). Changes in sea ice are also predicted to reduce feeding opportunities for polar bears and lead to declines in body condition and mass (Derocher et al. 2004). The result of these multiple stressors on polar bear health will likely impact their survival. Correlative analyses have suggested that elevated concentrations of contaminants negatively impact health of polar bears by altering concentrations of hormones, vitamins, and immune status (Bernhoft et al. 2000; Braathen et al. 2004; Skaare et al. 2001). These studies focused on the changes in health biomarkers associated with concentrations of lipophilic contaminants including polychlorinated biphenyls (PCBs), but the possible effects of heavy metals such as mercury (Hg) were not directly examined. The life history characteristics of ursids are known to influence both the concentrations of toxicants and many of the biomarkers assessed (Hellgren et al. 1990; Polischuk et al. 2002; Tomasi et al. 1998). The complicated variations in physiology and biochemistry among cohorts, therefore, require critical assessment before, or in parallel, with the assessment of the role of contaminants on polar bear health.
Biomarkers in toxicology are used to assess the potential responses of biological systems to contaminant exposure. Biomarkers have been categorized as biomarkers of exposure, biomarkers of effect, or biomarkers of susceptibility in comparisons between exposed (or gradients of exposures) and unexposed individuals, cohorts, or populations (Newmann 2010). The lack of unexposed or control (reference) animals in studies of free-ranging species makes these comparisons difficult, and researchers have relied on the use of correlative studies, a weight-of-evidence based approach, clinical diagnostics, or comparisons to threshold effect levels of health risks established for unrelated species (Letcher et al. 2010; Sonne 2010). The use of biomarkers as indicators of adverse biological impact, however, is complicated by the physiological mechanisms organisms use to maintain homeostasis and optimize metabolic and reproductive activities. For example, polar bears are adapted to the arctic climate by physiological and behavioral modifications such as delayed implantation, seasonal breeding, fasting during maternal denning, and extended periods of lactation (Amstrup 2003) that are known to be regulated by changes in the concentration of hormones, associated binding proteins, and co-factors (Hellgren 1998; Norris 2006; Tomasi et al. 1998). The natural variations of biomarkers due to non-toxicological mechanisms are largely unknown for most free-ranging species. Thus, dealing with confounding variables can be difficult and elusive. Furthermore, cross-sectional studies of free-ranging species cannot control for factors such as the time and dose of contaminants that ultimately led to the changes in the biomarkers assessed and may direct researchers to erroneous conclusions of adverse biological impacts.
Selenium (Se) supports many biological functions and also acts in the protection against toxicosis. Selenium is essential for the formation of many selenocysteine-containing proteins that regulate gonadal maturation, immune function, and the formation of thyroid hormones (Kohrle et al. 2005; Van Lente and Daher 1992). Seleno-proteins are also involved in biological activities in the brain, thyroid, and liver that limit oxidative damage of free radicals induced by aging, pathogens and contaminants (Berry and Ralston 2008; Khan and Wang 2010; Mayne 2003; Ralston and Raymond 2006; Scandalios 2005). Seleno-proteins reduce oxidative stress through non-enzymatic antioxidant activities that react with oxyradicals directly, and by enzymatic antioxidant activities that catalyze reactions and reduce the number of oxyradicals present. Non-enzymatic antioxidant activities, such as the sequestration of Hg by seleno-compounds, are thought to be the primary mechanism by which marine mammals tolerate high concentrations of dietary Hg (AMAP 2004a). An equimolar ratio (1: 1) between Se and Hg has been suggested in marine organisms to maintain detoxification via sequestration of Hg (Dietz et al. 2000; Woshner et al. 2002; Yoneda and Suzuki 1997a). Selenium binds to mercury in kidney and liver as mercuric selenide (HgSe), an inert end product of the detoxification of methylmercury by demethylation (Wagemann et al. 2000; Woshner et al. 2002; Khan and Wang 2010), whereas many mercury compounds in circulation are bound to selenoprotein P (Fairweather-Tait et al. 2010). Glutathione peroxidase (GPx) is a Se dependent protein that acts as an enzymatic antioxidant by reduction of potentially damaging hydrogen peroxides (Newmann 2010). Glutathione peroxidase activity has been reported to be altered by the bioaccumulation of Hg, although the exact mechanism is under debate (Brigelius-Flohe 1999; Carmagnol et al. 1983; Chen et al. 2006).
Thyroid hormones have been used as biomarkers of contaminant exposure as well as the assessment of overall health of marine and terrestrial animals (Rolland 2000; Rosa et al. 2007; Woshner et al. 2008). The biological actions of thyroid hormones include the regulation of metabolism, growth, cellular differentiation, and reproduction, as well as permissive actions that enable cells to exert an optimal response to other endogenous and exogenous stimuli (Cunningham and Klein 2007; Norris 2006). Many factors alter thyroid hormone production including age, sex, reproduction, temperature, diurnal and seasonal cyclicity, and nutrition. Concentrations of PCBs, polybrominated diphenyl ethers (PBDEs), and their metabolites have been associated with alterations in the thyroid hormone levels in polar bears and other species, but these interactions have not been consistent across studies (Braathen et al. 2004; Letcher et al. 2010; Skaare et al. 2001; Sonne 2010). Heavy metals such as Hg have also been implicated in the disruption of the hypothalamus-pituitary- thyroid (HPT) system (Rolland 2000), but have not been studied in polar bears. Thyroid status is determined by measuring the concentrations of bound and free fractions of thyroxine (T4) and tri-iodothyronine (T3) that are maintained at optimal levels through negative (and positive) feedback mechanisms to the pituitary (Figure 1). Disruption of thyroid function can occur through deficiencies in iodine or selenium, changes to the binding proteins in circulation, decreases in the transformation of T4 to T3, or disruption of feedback systems (Bruggemann and Darras 2009; McNabb 1995). Baseline data are needed on the selenium and thyroid hormone concentrations of Southern Beaufort Sea (SBS) polar bears to identify variations among cohorts, as well as the possible multiple and interactive effects of toxicants.
Figure 1.
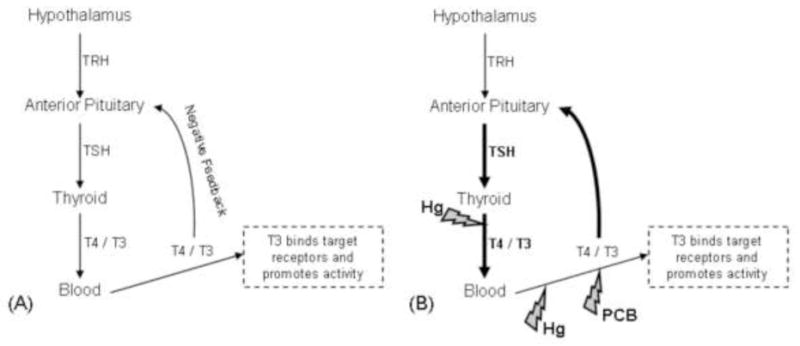
(A) Conceptual model of the homeostatic regulation of hypothalamus – pituitary – thyroid axis. When circulating thyroxine (T4) and tri-iodothyronine (T3) concentrations are within optimal limits, further production of T4 and T3 by the thyroid is inhibited. (B) Conceptual model of the response of the hypothalamus – pituitary – thyroid axis when exposed to contaminants. Mercury (Hg) reduces circulating concentrations of T4 and T3 through disruption of thyroid peroxidase activity during hormone synthesis in the thyroid and through competitive binding of transport protein albumin in the blood. Polychlorinated biphenyls (PCBs) reduce circulating concentrations of T4 and T3 through competitive binding of transport protein transthyretin. The hypothalamus – pituitary – thyroid axis responds to the reduction of circulating T4 and T3 concentrations by release of negative feedback mechanisms to the pituitary that signal an increase production of thyroid stimulating hormone (TSH) that consequently increases production of T4 and T3 by the thyroid.
In the present study, we question whether blood-based biomarkers of selenium and thyroid status were adequate measures of adverse biological effects of toxicants in polar bears. The biomarkers examined included whole blood and serum concentrations of selenium, glutathione peroxidase activity, thyroid hormone concentrations and ratios, and albumin concentrations. Our first objective was to report the levels of toxicants (Hg and PCBs) and biomarkers in SBS polar bears, describe variations among sex and age cohorts, and compare these to the known values reported in other polar bear sub-populations. The second objective was to examine whether selenium and thyroid status of polar bears was associated with circulating concentrations of Hg and PCB as predicted under the proposed mechanisms of toxicity for these chemicals individually and in combination, with consideration of the physiologic drivers of these biomarkers. These data are presented in the context of key cohorts (e.g., prime reproductive aged males, solitary females in estrous, and females caring for young) and the natural life history of the polar bear.
2. Materials and methods
2.1. Sample collection
Animal handling procedures were approved by animal care and use committees at the U.S. Geological Survey Research Program and the University of Alaska Fairbanks (UAF IACUC protocol 04-58). A total of 58 free-ranging SBS polar bears (31 males, 27 females) were captured by immobilization with Telazol (Warner–Lambert)-filled projectile darts fired from a helicopter during spring (March – May) 2007 in collaboration with the U.S. Geological Survey Ursid Research program as described previously (Bentzen et al. 2008; Cardona-Marek et al. 2009; Kirk et al. 2010). Ten females were accompanied by cubs (cubs of the year to 2 year olds). A vestigial premolar was removed from subadult and adult bears to estimate age by counting the cementum annuli (Matson Laboratory, Milltown, MT). Age of cubs of the year (COYs) through 3 year old animals was estimated by body size and inclusion in family group. Standard length (length) was recorded as the straight line length above the bear measuring the distance from the tip of the nose to the tip of the bony part of the tail while in sternal recumbency. Body mass (using a tripod, hoist, scale and net) was recorded for each bear. Body condition index (BCI) was determined from the natural log (ln) of mass and standard body length for each bear [BCI = (lnMass − 3.07*lnLength + 10.76)/(0.17 + 0.009 * lnLength); Cattet et al. 2002]. Blood samples were collected from either the femoral vein or artery into non-additive and K3 EDTA Vacutainers™ (BD Vacutainers, Preanalytical Solutions). Blood samples were prevented from freezing and non-additive tubes centrifuged within 6 hours of collection to separate serum from the packed cells (clotting proteins and blood cells). After gentle remixing, subsamples of blood collected into K3 EDTA tubes were transferred to cryogenic vials (Nalge Nunc International, Rochester, NY) or Teflon (fluoropolymer) vials (Savillex, Minnetonka, MN) for Hg and PCB analysis, respectively. Hematocrit (% packed cell volume) was estimated after transfer of whole blood into heparinized microhematocrit capillary tubes (Fisher Scientific, Pittsburgh, PA) and centrifugation for 3 minutes at 10,400 RPM (TRIAC Centrifuge 0200/0206, Becton, Dickinson, and Company). One cryogenic vial (~2 mL) remained unfrozen and transferred chilled (~4°C) to the Wildlife Toxicology Laboratory (WTL, University of Alaska Fairbanks) for analysis of glutathione peroxidase (GPx) activity. Samples for GPx activity were shipped by commercial airline two times per week (frequency dependent on capture and flight schedules).
2.2 Mercury (Hg) analysis
Whole blood was analyzed for the concentration of total Hg at the WTL. Concentrations of Hg were determined on a DMA-80 Direct Mercury Analyzer (Milestone Inc., Shelton, CT) using an eleven point calibration curve ranging from 1 to 400 ng (r2 = 0.998; method detection limit approximately 50 pg) and following modified Environmental Protection Agency methods. Standards were analyzed before unknown samples and after each set of 20 individual samples. Standard reference materials included freeze-dried human hair (IAEA-085, 0.573 mg/kg; IAEA-086, 23.2 mg/kg, International Atomic Energy Agency, Vienna, Austria), freeze-dried fish muscle homogenate (Lake Superior Standard Reference Material 1946, National Institute of Standards and Technology), and standard spiking solution (0.10 mg/kg, Perkin-Elmer, Waltham, Massachusetts). Percent recoveries of standard reference materials and spikes were within 88 – 110 %. The coefficient of variation for duplicate samples was less than 14%. Concentrations of Hg in whole blood are reported on a wet weight basis (Hg ng/g ww). Mercury concentrations in whole blood were also converted to molar concentrations (nmol/g; molecular weight of Hg = 200.59 g/mol) for comparison to molar Se concentrations.
2.3 Polychlorinated biphenyl (PCB) analysis
Procedures for PCB determination in whole blood followed modified procedures of Sloan et al. (2005). Briefly, 0.91 ± 0.12 g of whole blood was mixed with sodium sulfate and magnesium sulfate (1.5: 1, v/v) as drying agents, and extracted with dichloromethane (DCM) on an Accelerated Solvent Extractor (ASE 200, Dionex Corp., Sunnyvale, CA) at the WTL. Extracts were delivered to the NOAA/NMFS Montlake Laboratory in Seattle, WA. Each sample extract was filtered on gravity flow columns containing silica gel and alumina to remove interfering polar compounds and then further clean-up was conducted using size exclusion high performance liquid chromatography (SEC-HPLC). Individual PCB congeners were detected at NOAA/NMFS using a low-resolution quadrupole gas chromatograph/mass spectrometer (GC/MS) system equipped with a 60-meter DB-5 capillary GC column (0.25 mm i.d. and 0.25 μm film thickness) and a 10-meter guard column (0.53 mm i.d.). Each sample batch (n = 14) included a series of standards and were based on a five-point calibration curve. Percent recoveries were 98.7 ± 1.7%, 102.1 ± 1.9%, and 100.0 ± 15.9% for internal spike (PCB103) in blood/SRM/method blank, certified congeners from SRM, and congeners of duplicates, respectively. Method detection limits ranged from 0.07 – 0.79 ng/g (lowest of calibration range) and all method blanks were below detection. Samples with PCB concentrations below method detection limits were set at ½ the minimum detection limit (0.035 ng/g). Only 7 of the 40 PCB congeners examined, were detected in blood of polar bears (99, 105, 138, 153, 170, 180, and 194; ΣPCB7). These congeners were selected based on those found in ≥41% of animals sampled (threshold based on an apparent break in the proportion of animals having concentrations above detection as remaining congeners were present in < 7% of animals sampled). Lipid in whole blood was determined through detection and summation of five lipid classes (e.g., wax esters, triglycerides, free fatty acids, free cholesterol and phospholipids) using thin layer chromatography coupled with flame ionization detection (TLC/FID) on an Iatroscan Mark 6 (Iatron Laboratories, Tokyo, Japan, Shantha 1992). The concentration of ΣPCB7 was reported as ng/g wet weight (ng/g ww) and ng/g lipid weight (ng/g lw) of whole blood.
2.4 Selenium (Se) status
Estimates of Se status were determined through the analysis of Se concentrations in whole blood and serum, and glutathione peroxidase (GPx) activity. Concentrations of Se in both serum and whole blood were included because these values have not been previously reported for free-ranging polar bears, and to assess whether concentrations of Se in serum represented more acute changes in Se status versus whole blood. All assays were performed at the WTL. Samples for Se analysis were heated using a two step digestion in a Perkin Elmer Multiwave 3000 microwave oven. Whole blood and serum (0.350 ± 0.076 g) were placed in a 3:1 nitric acid: hydrogen peroxide (v/v) mixture and heated to 170 °C for 15 minutes. The initial digest was diluted to 20 mL with ultrapure water (NANOpure Model D4751, Barnstead International, Dubuque, IA). A sub-sample of the diluted digest (2 mL) underwent a secondary digest (heated to 95 C for 60 minutes) with excess hydrochloric acid (HCl) (1:1 v/v) to reduce Se (VI) to Se (IV). Quality control samples (blanks, spikes, duplicates, matrix spikes, and standard reference materials) were included in each digestion batch. Standard reference materials included Lake Superior Fish Tissue 1946 (0.491 ± 0.043 mg Se/kg, National Institute of Standards and Technology (NIST), Gaithersburg, MD) and DOLT-4 (8.3 ± 1.3 mg Se/kg; Dogfish Liver Certified Reference Material for Trace Metals, National Research Council Canada, Institute for National Measurement Standards, Ottawa, Ontario, Canada). Selenium concentrations were determined using a 7 point calibration curve (0.04 to 4.48 ng/g) through mercury/hydride system - flame ionization atomic spectrometry (MHS-FIAS) on a Perkin Elmer AAnalyst 800 atomic absorption spectrometer (AAS). Sodium borohydride (0.2% NaBH4 in 0.05% NaOH) was used as the reductant in a 10% HCl carrier solution. The mean percent recovery of quality control samples were 113%, 114%, 100%, 126%,, and 119% for spikes, sample spikes, duplicates, Lake Superior Fish SRM, and DOLT4 SRM, respectively. Selenium in serum and whole blood are reported in ng/g ww and as molar concentrations (molecular weight of Se = 78.96 g/mol).
Whole blood GPx activity was measured by a modified procedure described by Carmagnol et al. (1983) using a refrigerated Eppendorf 5810R spectrophotometer (DU Series 520; Beckman Instruments, Inc., Fullerton, CA). Whole blood samples delivered to the WTL were centrifuged at 1500 g to separate erythrocytes from plasma. Erythrocytes (packed cells) were washed twice in cold 0.9% NaCl and lysed in 4 volumes of ultrapure water. The hemolysate was isolated through centrifugation at 10,000 g. Hemoglobin (Hgb) concentrations of the packed cells were determined using Drabkin’s Reagent (Sigma-Aldrich Corp., St. Louis, MO). This procedure is based on the oxidation of hemoglobin to methemoglobin (MetHgb) by potassium ferricyanide, which reacts with potassium cyanide to form cyanMetHgb, and has a maximum absorbance at 540 nm. Hemoglobin concentration of the hemolysate (mg Hgb/mL hemolysate) was determined through comparison to a calibration curve [5–20 mg Hgb/mL; 100 mg Protein (bovine erythrocytes) Hemoglobin Ao Ferrous; Sigma-Aldrich Corp., St. Louis, MO] and multiplied by the dilution factor. Total hemoglobin content of whole blood was estimated by multiplying the concentration of hemoglobin in the hemolysate by the percent hematocrit (mL packed cells/100 mL whole blood) estimated in the field. Total hemoglobin content of whole blood was reported as g Hgb/dL whole blood.
GPx activity was determined using the Glutathione Peroxidase Cellular Activity Assay Kit (Sigma-Aldrich Corp., St. Louis, MO). This kit included a phosphate buffer, NADPH (B NADPH; B-Nicotinamide Adenine Dinucleotide Phosphate, Reduced) assay reagent, and 30 mM tert-butyl hydrogen peroxide solution. A GPx standard was used to ensure instrument and procedural accuracy (100 units diluted to 0.25 units/mL in phosphate buffer; Sigma-Aldrich Corp.). The kit measures GPx activity through an indirect method based on the oxidation of glutathione (GSH) to oxidized glutathione (GSSG) catalyzed by GPx, which is then coupled to the recycling of GSSG back to GSH utilizing glutathione reductase (GR) and NADPH. The method employed detection of decreased NADPH absorbance at 340 nm (initial delay of 15 seconds, followed by 6 readings at 10 second intervals), which is indicative of GPx activity, since GPx is the rate-limiting step for the reaction (per product info sheet, Sigma-Aldrich Corp.). Analysis was performed on duplicate samples. GPx activity in this method, therefore, only includes selenium dependent enzymes located within erythrocytes and was calculated as:
where, DF = assay dilution factor = 10; 6.22 = emM for NADPH (extinction rate); 0.05 mL = amount of erythrocyte lysate. GPx Units/mL were divided by the hemoglobin content of the hemolysate (mg Hgb/mL hemolysate) to determine final GPx activity:
2.5 Thyroid status
Thyroid hormone concentrations (total thyroxine, TT4; free thyroxine, FT4; total triiodothyronine, TT3; free triiodothyroinine, FT3) in serum were determined at Michigan State University Pathobiology and Diagnostic Investigation Center following published procedures (Rosa et al. 2007). Validations of thyroid assays for use in ursids were performed through a serial dilution of 2 pooled samples. Serial dilutions of polar bear serum added to the zero standard or protein buffer of the radioimmunoassay were 92%, 97%, 92%, and 99% the projected concentrations of TT4, TT3, FT4, and FT3, respectively. Inter-assay and intra-assay correlations of variations (CVs) ranged from 8 – 24 % with good parallelism (correlation mean > 0.9) between dilution of pooled samples and standard dilutions in protein buffer. Concentrations of hormones (total fractions, nmol/l; free fractions, pmol/l) and molar ratios (TT4: FT4, TT3: FT3, TT4: TT3, FT4: FT3) were reported (molecular weight of T3 = 650.97 g/mol; molecular weight of T4 = 776.87 g/mol).
Albumin concentration was determined using a serum chemistry comprehensive panel on a DRI-CHEM® Veterinary Chemistry Analyzer (Heska Corp.) at the UAF. A human serum reference sample (Heska ® Chemistry Control, Heska, Corp.) with known amounts of albumin was measured to ensure procedure and instrument accuracy.
2.6 Statistics
Cohorts were based on sex, age, and natural life history characteristics (dependent young, rapid growth rate, reproductive status, and lactational demand) as used previously for polar bears (Amstrup 2003; Kirk et al. 2010; Rode et al. 2010; Tryland et al. 2002). Cohorts included: young, ages 1 – 5 years; prime reproductive age males, 6 to 15 years; prime reproductive age females, 6 to 15 years; and older animals, ≥ 16 years of age without cubs. Females were further grouped as solitary or with dependent cubs. Two 14-year-old females had cubs of the year, and one 24-year-old female had 2 yearling cubs. Thus, the older animal cohort was considered to be ≥16 years of age and without cubs (rather than ≥ 13 years of age used previously in Tryland et al. 2002). The young cohort included both dependent young (ages 1 – 2, n = 5) and sub- adults (ages 3 – 5, n = 4) due to the small number of young animals sampled, and because both were considered to be in a rapid growth phase. Because the young cohort was not sexually mature, the gender of this cohort was not considered a significant variable in analyses.
Statistics were performed using Systat 11 (Systat Software Inc.). As all PCB congeners are considered to be readily bioavailable in blood regardless of the association with lipids, wet weight concentrations of PCBs were used to assess relationships. Variables were log transformed as needed to meet normality criteria and to improve distributional properties. Log transformations did not improve the distribution of whole blood Se: serum Se ratio and albumin concentrations, and were thus examined with no transformation. Analysis of covariance (ANCOVA) was used to assess differences in biomarkers between sexes with age as a covariate. Analysis of variance (ANOVA) was used to assess differences between biomarkers and toxicants by cohort. Pearson’s correlations were used to assess correlations between toxicants, biomarkers, and body condition index. Generalized linear models (GLM) were used to assess whether biomarkers of Se status and thyroid status were related to the concentrations of toxicants (Hg, PCBs) while controlling for the effect of sex (i.e., sex was used as a covariate). Body condition index (BCI) was removed from these models due to the lack of significance (p > 0.3). Significant differences were determined to be at p < 0.05.
Biomarkers of thyroid status were expected to relate to each other because of the feedback mechanisms inherent to the HPT axis. A correlation-based principal components analysis (PCA) with varimax rotation, therefore, was used to investigate simultaneous relationships between toxicants (Hg, ΣPCB7) and measures of thyroid status (total T4, free T4, total T3, free T3, and albumin) by prime aged cohorts (males, solitary females, and females with cubs). Absolute values of component loadings of ≥ 0.6, 0.3 – 0.5, and ≤0.3 were used to describe whether relationships among variables were strongly, moderately, or weakly correlated, respectively. Signs (+ or −) between variables indicated whether these relationships were positively or negatively correlated. Young and older cohorts were excluded from these analyses due to low sample size. Variables that were strongly correlated by PCA, were further explored using GLM and Pearson’s pairwise comparisons.
3. Results
3.1 Concentrations of circulating toxicants
Concentrations of Hg and ΣPCB7 in whole blood (ng/g ww) were positively correlated in males (p < 0.001), but unrelated in females (solitary females and females with cubs combined, p = 0.834). Concentrations of Hg in whole blood ranged from 10.25 to 228.05 ng/g ww and were similar between cohorts (SEM ± SD; solitary females, 69.8 ± 65.2 ng/gww; females with cubs, 41.6 ± 29.0 ng/g ww; males, 38.2 ± 18.4 ng/g ww; young, 52.4 ± 14.7 ng/g ww; older animals, 63.2 ± 44.9 ng/g ww; ANOVA, f = 1.383, p = 0.253; Figure 2A). Concentrations of blood ΣPCB7 ranged from 2.03 to 132.8 ng/g ww (568 to 23,200 ng/g lw). The concentrations of individual PCB congeners were correlated (r = 0.47, p < 0.005), and 53% of the ΣPCB7 consisted of PCB 153. Wet weight and lipid weight concentrations of ΣPCB7 were significantly greater in females and young compared to males (solitary females ≥ females with cubs ≥ young > males; SEM ± SD; females with and without cubs, 26.4 ± 25 82 ng/g ww; young, 26.8 ± 10.7 ng/g ww; males, 8.9 ± 5.8 ng/g ww; ΣPCB7 ww, ANOVA, f = 12.255, p < 0.001, Figure 2A; ΣPCB7 lw, ANOVA, f = 5.467, p < 0.001). Elevations in ΣPCB7 were inversely correlated with BCI (r = −0.675, p < 0.001), and body condition index was lowest among females and young (ANOVA, f = 13.145, p < 0.001, Figure 2B). There was no relationship between Hg and BCI (p > 0.9).
Figure 2.
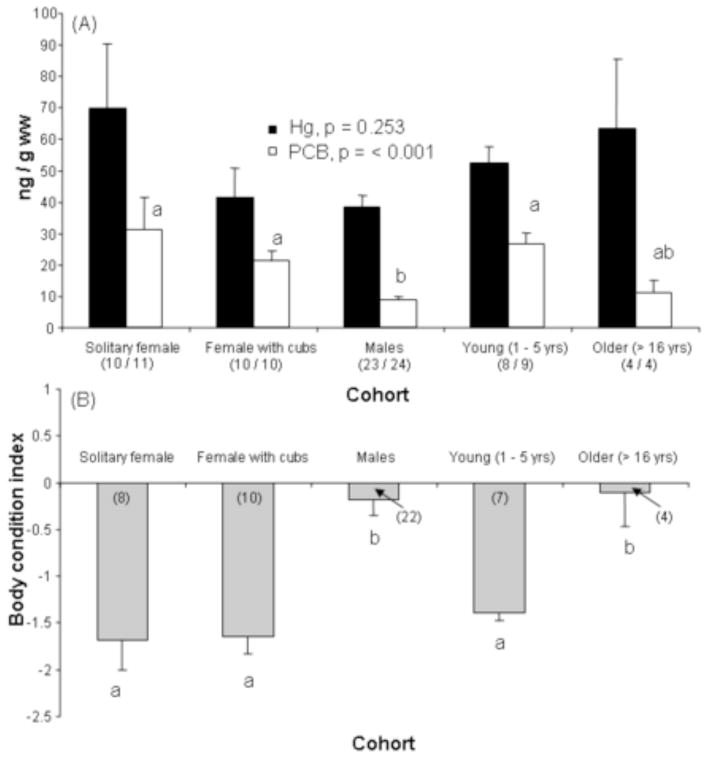
(A) Wet weight concentrations of circulating mercury (Hg ng/g ww) and polychlorinated biphenyls (ΣPCB7; ng/g ww) in free ranging polar bears by cohort. (B) Body condition index of polar bears by cohort. Data are shown as mean ± standard error. Mean differences examined by analysis of variance (ANOVA) on log transformed data. The number of animals in each cohort is listed in parentheses.
3.2 Biomarkers of selenium and thyroid status
Selenium concentrations in whole blood were significantly greater in males versus females (whole blood Se; males, 536.2 ± 23.4 ng/g ww; females, 459.1 ± 16.2 ng/g ww; ANCOVA, f = 5.956, p = 0.019; age as a covariate, f = 0.482, p = 0.492; Table 1). Whole blood Se and serum Se concentrations were correlated (r = 0.488, p = 0.001), with whole blood Se generally two times greater than serum Se. The range of GPx activity varied over 2 fold among individual bears (range 109.31 – 206.84 mUnits GPx/mg Hgb), and increased as follows: males and older ≤ young ≤ solitary females ≤ females with cubs (Table 1; Figure 3).
Table 1.
Selenium (whole blood and serum) concentrations, glutathione peroxidase (GPx) activity, hemoglobin concentration, and percent hematocrit in the blood of male and female polar bears. Data are shown as mean ± standard deviation, range and median. Ratios were calculated using molar concentrations. Analysis of covariance performed on log transformed data. Significant differences are shown in bold.
| Males | Females | Sex | Age | |
|---|---|---|---|---|
| n = 24 – 29* | n = 20 – 26* | f, p | f, p | |
| Whole blood Se, pmol/g | 42.34 ± 9.78 | 36.25 ± 6.27 | 5.956, 0.019 | 0.482, 0.492 |
| 28.02 – 66.24 | 26.25 – 46.88 | |||
| 40.77 | 36.25 | |||
| Serum Se, pmol/g | 20.26 ± 3.09 | 19.51 ± 4.04 | 0.662, 0.420 | 0.015, 0.902 |
| 14.88 – 25.65 | 13.22 – 29.45 | |||
| 20.96 | 19.81 | |||
| Whole blood Se: Serum Se | 2.10 ± 0.42 | 1.94 ± 0.39 | 2.298, 0.137 | 0.344, 0.561 |
| 1.42 – 3.14 | 1.30 – 2.74 | |||
| 2.14 | 1.94 | |||
| Whole blood Se: Whole blood Hg | 6.38 ± 3.62 | 5.24 ± 4.19 | 2.207, 0.146 | 1.102, 0.300 |
| 2.49 – 17.84 | 1.02 – 18.94 | |||
| 5.07 | 4.03 | |||
| Serum Se: Whole blood Hg | 3.01 ± 1.46 | 2.74 ± 2.13 | 1.102, 0.300 | 1.709, 0.199 |
| 1.13 – 7.02 | 0.46 – 10.52 | |||
| 2.60 | 2.13 | |||
| GPx, mUnits/mg Hb† | 143.71 ± 18.99 | 155.16 ± 18.99 | 3.106, 0.086 | 0.129, 0.722 |
| 109.31 – 194.41 | 121.75 – 206.84 | |||
| 143.09 | 155.51 | |||
| Hemoglobin, g/dL whole blood | 15.99 ± 3.70 | 13.97 ± 2.85 | 3.232, 0.080 | 0.057, 0.813 |
| 10.21 – 25.96 | 10.27 – 22.43 | |||
| 15.77 | 13.26 | |||
| Hematocrit, percent | 44.21 ± 5.16 | 42.06 ± 4.20 | 2.260, 0.141 | 0.056, 0.814 |
| 31.50 – 54.70 | 33.50 – 49.50 | |||
| 44.50 | 41.88 |
sample size for GPx, Hb, and hematocrit was 24 for males and 20 for females;
Hb = hemoglobin
Figure 3.

Glutathione peroxidase (GPx) activity (mUnits/mg hemoglobin) of polar bears by cohort. Data are shown as mean ± standard error. Mean differences examined by analysis of variance (ANOVA) on log transformed data. Hb = hemoglobin. The number of animals in each cohort is listed in parentheses.
Concentrations of thyroid hormones (TT4, FT4, TT3, FT3) were greater in females than males (Table 2). Only FT3 concentrations exhibited a significant decrease with age. The ratio of total to free concentrations of thyroxine (TT4: FT4) was similar among sex and age cohorts. The ratio of total T4 to total T3 (TT4: TT3) varied by 4 fold among individuals, but mean values did not differ by sex or age. Males exhibited a greater mean value and range in the ratio of total to free concentrations of tri-iodothyronine (TT3: FT3) and in the ratio of free T4 to free T3 (FT4: FT3). Concentrations of albumin were greater in males than females (Table 2).
Table 2.
Thyroid hormone concentrations, thyroid hormone molar ratios, and albumin concentrations of male and female polar bears.
| Males | Females | Sex | Age | |
|---|---|---|---|---|
| n = 28 | n = 24 | f, p | f, p | |
| TT4, nmol/l | 10.89 ± 4.33 | 19.30 ± 6.82 | 25.06, <0.001 | 0.99, 0.326 |
| 4.0 – 23.0 | 11.0 – 32.0 | |||
| 10.50 | 18.50 | |||
| FT4, pmol/l | 6.68 ± 2.18 | 10.75 ± 2.64 | 32.99, <0.001 | 0.046, 0.832 |
| 4.0 – 11.0 | 7.0 – 17.0 | |||
| 6.00 | 10.00 | |||
| TT3, nmol/l (excludes 1 outlier) | 1.12 ± 0.35 | 1.62 ± 0.45 | 21.08, <0.001 | 1.98, 0.166 |
| 0.30 – 2.10 | 0.90 – 2.50 | |||
| 1.10 | 1.55 | |||
| FT3, pmol/l | 0.37 ± 0.28 | 0.72 ± 0.35 | 24.40, <0.001 | 6.55, 0.014 |
| 0.10 – 1.30 | 0.3 – 1.8 | |||
| 0.30 | 0.70 | |||
| TT4:FT4† | 1.61 ± 0.29 | 1.77 ± 0.33 | 1.48, 0.229 | 3.24, 0.079 |
| 1.0 – 2.2 | 1.2 – 2.5 | |||
| 1.60 | 1.71 | |||
| TT3:FT3† | 3.85 ± 1.65 | 2.46 ± 0.55 | 15.73, <0.001 | 4.39, 0.042 |
| 1.40 – 8.00 | 1.39 – 3.67 | |||
| 3.33 | 2.40 | |||
| TT4:TT3 | 10.00 ± 3.42 | 12.53 ± 5.49 | 3.16, 0.082 | 0.82, 0.370 |
| 5.00 – 20.91 | 7.12 – 30.0 | |||
| 9.62 | 11.62 | |||
| FT4:FT3 | 24.21 ± 13.21 | 17.78 ± 8.40 | 6.15, 0.017 | 10.89, 0.002 |
| 7.69 – 60.00 | 6.67 – 43.33 | |||
| 20.00 | 15.36 | |||
| Albumin, g/dl | 4.34 ± 0.34 | 4.10 ± 0.29 | 6.010, 0.018 | 0.069, 0.794 |
| 3.90 – 5.00 | 3.30 – 4.50 |
Data are shown as mean ± standard deviation, and median. Analysis of covariance performed on log transformed data except for albumin that was used as is. Significant differences are shown in bold.
value * 103
3.3 Relationships among biomarkers of selenium status, thyroid status and toxicant concentrations
Concentrations of Se (whole blood and serum) and GPx activity were significantly positively correlated with circulating concentrations of Hg in prime aged polar bears (Table 3; Figure 4A–C). GPx activity was also significantly negatively related to the Se: Hg molar ratio (Figure 4D). Concentrations of T3 were significantly positively related to the concentrations of Hg even after controlling for the variation by sex (Table 3; Figure 5A). TT3 levels were significantly positively related to the concentrations of Se, and significantly negatively related to the Se: Hg molar ratio (Figure 5B and C).
Table 3.
Analysis of variance tables from general linear regression models (GLM) of the relationships between biomarkers and whole blood concentrations of toxicants in prime aged (6–16 years) polar bears Analyses were performed on log transformed data for all biomarkers and toxicants, except for albumin which was used with no transformation. Se = selenium; GPx = glutathione peroxidase activity; TT4 = total thyroxine, TT4:TT3 = molar ratio of total thyroxine to total tri-iodothyronine; FT4: FT3 = molar ratio of free thyroxine to free tri-iodothyronine. Values highlighted in bold indicate a significant relationship at p < 0.05.
| Variable | f-value | P value | R2 |
|---|---|---|---|
| Whole blood Se (n = 34) | |||
| Sex | 4.702 | 0.038 | 0.419 |
| Hg | 10.329 | 0.003 | |
| ΣPCB7 | 0.539 | 0.472 | |
| Serum Se (n = 35) | |||
| Sex | 2.221 | 0.146 | 0.264 |
| Hg | 7.518 | 0.010 | |
| ΣPCB7 | 0.039 | 0.845 | |
| GPx (n = 28) | |||
| Sex | 1.449 | 0.240 | 0.340 |
| Hg | 7.518 | 0.031 | |
| ΣPCB7 | 0.039 | 0.845 | |
| TT4 (n = 36) | |||
| Sex | 7.349 | 0.011 | 0.429 |
| Hg | 0.155 | 0.697 | |
| ΣPCB7 | 1.503 | 0.229 | |
| TT3 (n = 36) | |||
| Sex | 6.974 | 0.013 | 0.470 |
| Hg | 8.608 | 0.006 | |
| ΣPCB7 | 0.305 | 0.585 | |
| FT4 (n = 36) | |||
| Sex | 12.434 | 0.001 | 0.468 |
| Hg | 0.783 | 0.383 | |
| ΣPCB7 | 0.248 | 0.622 | |
| FT3 (n = 36) | |||
| Sex | 7.731 | 0.003 | 0.444 |
| Hg | 6.350 | 0.017 | |
| ΣPCB7 | 0.153 | 0.699 | |
| TT4: TT3 (n = 36) | |||
| Sex | 1.352 | 0.253 | 0.196 |
| Hg | 2.493 | 0.124 | |
| ΣPCB7 | 0.933 | 0.341 | |
| FT4: FT3 (n = 36) | |||
| Sex | 0.783 | 0.383 | 0.199 |
| Hg | 5.254 | 0.029 | |
| ΣPCB7 | 0.015 | 0.903 | |
| Albumin (n = 36) | |||
| Cohort | 2.756 | 0.107 | 0.290 |
| Hg | 0.015 | 0.905 | |
| ΣPCB7 | 1.390 | 0.247 | |
Figure 4.
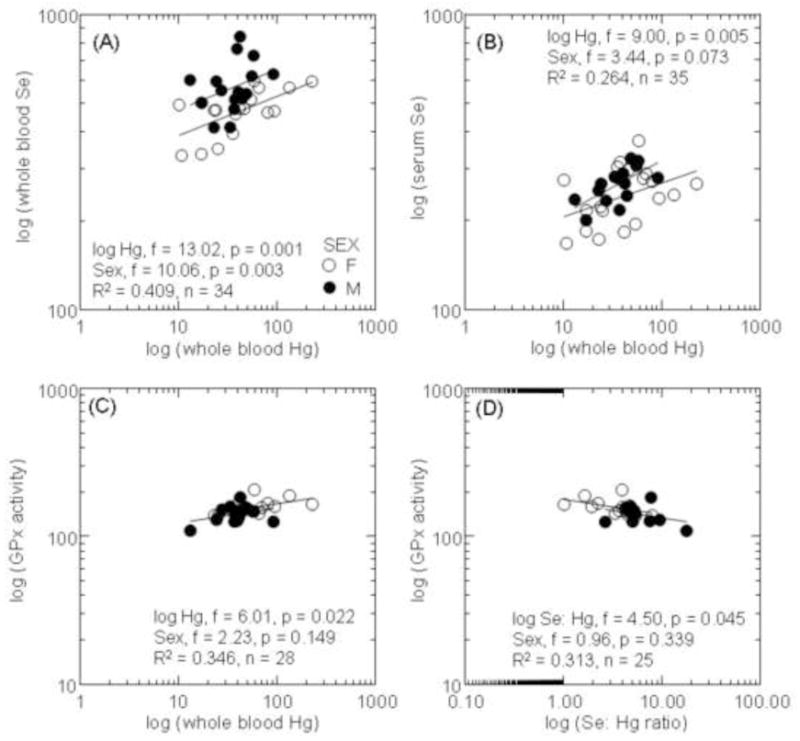
Concentrations of selenium (Se; ng/g ww) in whole blood (A) and serum (B) in relation to the concentrations of Hg in blood of prime aged polar bears. Glutathione peroxidase activity (mUnits/mg Hb) in relation to concentrations of Hg (C) and the molar ratio of Se: Hg (D) in blood of prime aged polar bears. General linear models were used to assess relationships between biomarkers and toxicants with sex as a covariate.
Figure 5.
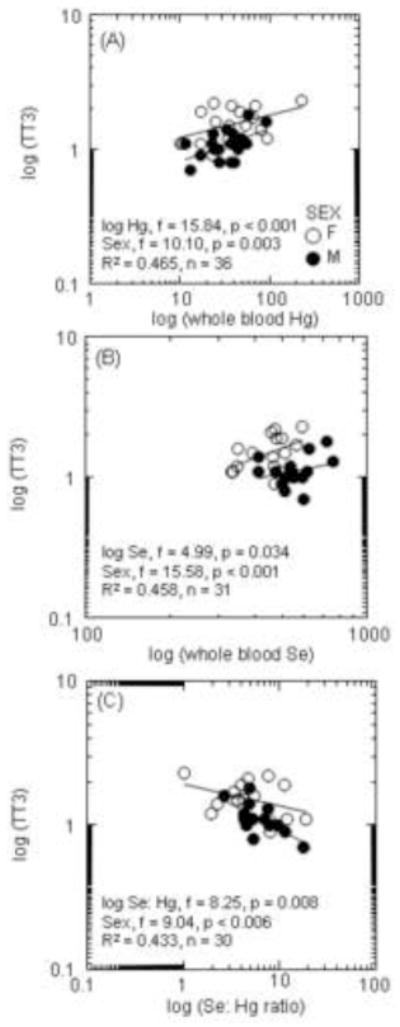
Concentrations of total tri-iodothyronine (TT3) and the concentration of Hg (A), whole blood Se (B), and the Se:Hg ratio (C) in prime aged polar bears. General linear models were used to assess relationships between biomarkers and toxicants with sex as a covariate.
The first two components of the correlation-based PCA represented 73 – 82% of the total variation in the variables examined (Figure 6; Table 4). The individual or combined concentrations of toxicants explained less than 39% of this variation (represented by Factor 2, Figure 6A–C; Table 4). Strongly positive correlations between Hg and ΣPCB7 were found in males (component loading for Hg, 0.897; component loading for PCB, 0.835) and solitary females (component loading for Hg, 0.789; component loading for PCB, 0.692; Figure 6A and B), whereas the concentrations of toxicants were inversely correlated in females with cubs (component loading for Hg, 0.777; component loading for PCB, −0.949; Figure 3C; Table 4). There was a strong negative correlation between toxicant concentrations and albumin in males (component loading relative to toxicants, −0.624). Further exploration of this relationship using general linear models suggested a significantly positive relationship between albumin and thyroxine, and a significantly negative relationship between albumin and PCBs among all prime age animals (Figure 7A and B). The correlational-based PCA indicated a strong negative correlation between toxicants and thyroxine (total and free fractions) in solitary females (component loadings relative to toxicants for TT4 and FT4, −0.948 and −0.848; Figure 6B; Table 4) that was further supported by examination of TT4 and toxicant correlations by physiological state (Figure 8A and B).
Figure 6.
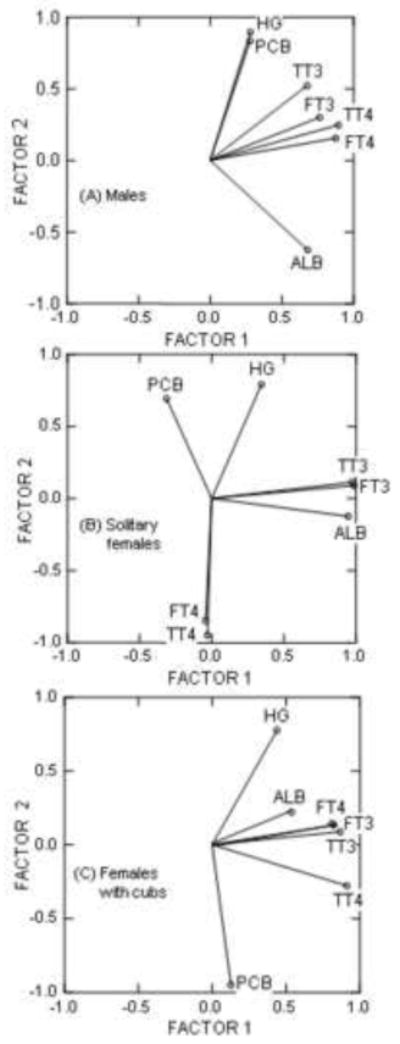
Correlation based principal components analysis of the relationships between thyroid status and toxicants in prime aged males (A), prime aged solitary females (B), and prime aged females with cubs (C). Statistics performed on log transformed data, except for albumin concentrations which were included as is.
Table 4.
Relationships between toxicants and biomarkers of thyroid status in polar bears by prime aged (6 – 15 years) cohorts. Component loadings were assessed using a correlation based principal components analysis. Values highlighted in bold are were strongly correlated (≥ 0.6). Signs (+ or −) in each column reflect whether the variable was positively or negatively correlated with the other variables in that column (i.e., relative to other variables making up that component loading).
| Prime Aged Males | Prime Aged Solitary Females | Prime Aged Females with Cubs | ||||
|---|---|---|---|---|---|---|
| Loadings for Factor 1 | Loadings for Factor 2 | Loadings for Factor 1 | Loadings for Factor 2 | Loadings for Factor 1 | Loadings for Component 2 | |
| PCB | 0.278 | 0.835 | −0.312 | 0.692 | 0.127 | −0.949 |
| Hg | 0.280 | 0.897 | 0.346 | 0.789 | 0.439 | 0.777 |
| TT4 | 0.894 | 0.247 | −0.029 | −0.948 | 0.912 | −0.278 |
| TT3 | 0.676 | 0.523 | 0.976 | 0.112 | 0.868 | 0.088 |
| FT4 | 0.876 | 0.156 | −0.042 | −0.848 | 0.824 | 0.134 |
| FT3 | 0.763 | 0.301 | 0.986 | 0.088 | 0.809 | 0.144 |
| Albumin | 0.679 | −0.624 | 0.947 | −0.125 | 0.537 | 0.222 |
| % total variation | 55 % | 33 % | 43 % | 39 % | 49 % | 24 % |
Figure 7.
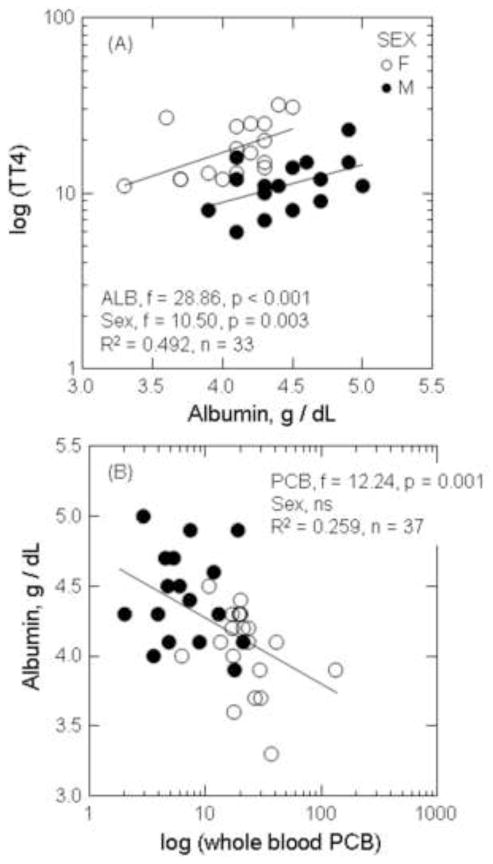
The relationship between concentration of total thyroxine (TT4) and albumin (A), and albumin and PCBs (B) in prime aged polar bears. General linear models were used to assess relationships between biomarkers and toxicants with sex as a covariate.
Figure 8.
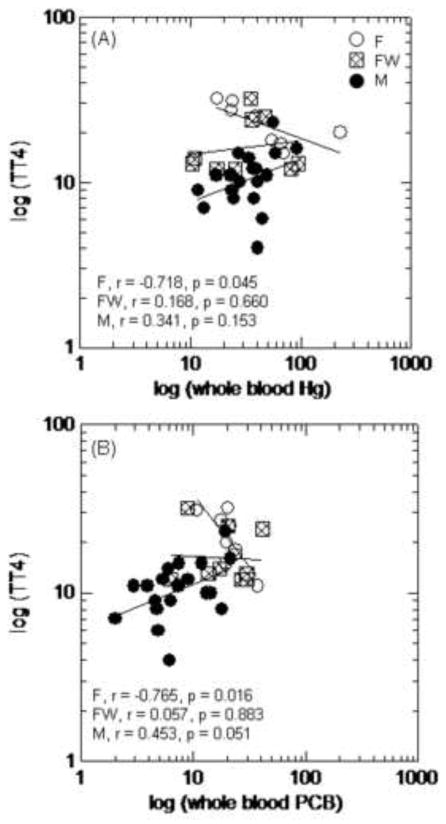
Correlations between total thyroxine (TT4) concentrations and the concentration of Hg (A) and the concentration of PCBs (B) in solitary females (F), females with cubs (FW), and adult males (M). Correlations examined using Pearson’s pairwise correlations on log transformed data.
4. Discussion
This study indicated that the concentrations of circulating toxicants differed among the physiologic states of Southern Beaufort Sea (SBS) polar bears. For example, we document that concentrations of Hg and ΣPCB7 were positively correlated in males and solitary females. Concentrations of Hg and ΣPCB7, however, were negatively correlated and more variable among females with cubs. Circulating Hg concentrations can be used as a proxy of recent feeding as Hg compounds are bioavailable to blood shortly after prey consumption and the half-life of circulating Hg is approximately 30 days (Klaassen 2001). The low Hg concentrations of many of the females with cubs, therefore, suggest that these animals had reduced feeding relative to other polar bears or that Hg was transferred to offspring via lactation. Previous studies found that diet as estimated by stable isotope values of δ15N and δ13C were strong predictors of Hg and PCB exposure to SBS polar bears (Bentzen et al. 2008; Cardona-Marek et al. 2009). The positive correlation between Hg and ΣPCB7 suggests that males and solitary females were exposed to these contaminants concomitantly during feeding. These data are consistent with the high Hg and PCB concentrations in ringed seals (Pusa hispida; Dehn et al. 2005; Kucklick et al. 2002) reported to be the primary prey of polar bears in this region. Concomitant exposure to lipophilic and heavy metal contaminants has not been previously documented in SBS polar bears, but must be considered when assessing potential adverse biological responses.
Wet weight and lipid-adjusted concentrations of circulating PCBs were greater in prime aged females (with and without cubs) compared to males, and positively correlated to reduced body condition scores. Females recently emerging from maternity dens have undergone fasting, and all females with cubs have high energy demands to maintain lactation for offspring (Atkinson and Ramsay 1995). Lower body condition scores among females versus males suggest that females mobilized a greater amount of lipids from body storage sites than males, resulting in the greater release of lipophilic contaminants to circulation. Similar changes in lipophilic toxicants in blood and subcutaneous adipose have been reported in polar bears and arctic fox (Alopex lagopus) and were related to lactation, recent increase in energy demand, or fasting (Fuglei et al. 2007; McKinney et al. 2009; Polischuk et al. 2002). The different disposition dynamics of heavy metal and lipophilic contaminants of females compared to males may also relate to the different feeding behaviors and sea-ice preferences among polar bear cohorts during late winter/early spring as females are more likely to remain closer to shore, especially if they have dependent young (Amstrup et al. 2000). Previous reports hypothesized that lipid soluble contaminants would be lower in females relative to males due to the gestational and lactational transport of contaminants to offspring (AMAP 2004b). Although this finding may be true for concentrations of toxicants stored in subcutaneous lipid and visceral tissues, this hypothesis does not appear to hold true for the circulatory disposition of toxicants in blood as presented in this study. Polar bears in the SBS are expected to undergo shortened feeding periods and greater energy expenditures to access prey under the proposed future changes in arctic sea ice (Derocher et al. 2004; Molnar et al. 2010). Further understanding of the disposition of contaminants is needed as blood concentrations of toxicants represent the most recent influence to target sites of toxicity, and most closely match the temporal window of biomarkers measured in blood.
Circulating concentrations of Se in polar bears during spring were greater in males than females, and equal or in molar excess to concentrations of blood Hg. Circulating Se concentrations largely reflect dietary exposure to Se-containing foods (Fairweather-Tait et al. 2010), although Se is also stored in tissues of polar bears (Woshner et al. 2001). The variation of circulating Se among individual bears, therefore, is largely a consequence of the location, species, and proportion of prey tissues recently consumed. Dietary sources of Se for SBS polar bears would be largely from the consumption of ringed seals. The molar ratio of Se: Hg in ringed seal muscle ranged from 3.2: 1 to 5.7: 1, and 72 % of the total Hg measured was in its methylated form (Woshner et al. 2001). Visceral tissues such as kidney and liver of ringed seal contained more inorganic forms of Hg and equimolar ratios of Se: Hg (Dietz et al. 2000; Woshner et al. 2001). The majority of Se in plasma of mammals is part of selenoprotein P which contains several selenocysteines and thus has high binding affinity for heavy metals (Fairweather-Tait et al. 2010; Yoneda et al. 1997; Yoneda and Suzuki 1997). Mercury forms also bind to a number of other seleno-compounds including bis(methlmercuric)selenide, selenomethioneine, free selenocysteines, and mercuric selenide (tiemanite) (Deitz et al. 2000; Woshner et al. 2001; Kahn and Wang 2010), as well as hemoglobin and albumin (Clarkson et al. 2007). The positive relationship between circulating concentrations of Se and Hg support the hypothesis that Hg in the blood of polar bears is bound, in part, to seleno-compounds. Molar ratios of Se: Hg (up to 19: 1 in the present study) above those found in common diet tissues of polar bears suggest that seleno-compounds in blood may also be retained in circulation by Hg. The negative relationships between TT3 concentrations and the Se: Hg molar ratios support a possible disruption in seleno-protein activities. Seleno-compounds are proposed to limit Hg toxicity through sequestration, Se-aided demethylation of MeHg, and inhibition of damaging free radicals; however, seleno-compounds also distribute dietary Hg to sensitive target organs such as the brain, liver, and kidney (Kahn and Wang 2010). Elevated Se: Hg molar ratios in the blood of polar bears likely limit oxidative stress initially induced by Hg, and aid in the distribution and elimination of Hg-compounds.
Glutathione peroxidase activity in polar bears increased with elevated concentrations of Hg. The negative correlation between GPx activity and the Se: Hg molar ratio in blood suggests that GPx may protect against Hg induced oxidative damage when Se: Hg ratios are low. This result is in agreement with other studies of Se status where the proportions of selenium forms measured in blood resulted from the bioavailability of dietary selenium, and the saturation thresholds of the various selenoproteins in circulation (Fairweather-Tait et al. 2010). Circulating concentrations of Hg in Hg-exposed humans was associated with an increase in both selenoprotein P and glutathione peroxidase, but the percentage of Hg associated with selenoprotein P increased with increasing concentrations of blood Hg (Chen et al. 2006). Changes in the concentrations or proportions of specific selenoproteins in response to toxicant exposure have not been examined in polar bears. Furthermore, the bioavailability of dietary Se and the biological saturation points of seleno-compounds are unknown. The elevated GPx activity in female polar bears with cubs suggest that factors such as recent denning or lactation may also increase oxidative stress. A similar increase of enzymatic antioxidants, including GPx, were also reported in peripartum cattle having low body condition scores and increased lactational demand (Bernabucci et al. 2005). It is assumed that a 1: 1 Se: Hg molar ratio in tissues is biologically inert and has no adverse effect (Khan and Wang 2010). Our data suggest that as Se: Hg ratios approach 1, enzymatic antioxidants such as GPx become more important in the sequestration of Hg and the reduction of free radicals by toxicants and other stressors.
The albumin concentrations of polar bears in the present study are considered to be within the normal range for free-ranging polar bears during spring. The concentrations of albumin reported for SBS polar bears in the present study (range 3.3 – 5.0 g/dl) were similar to the albumin concentrations for the presumably healthy free-ranging polar bears sampled in Svalbard (albumin, range 4.3 – 5.1 g/dl; Tryland et al. 2002). Albumin proteins in circulation have several biological roles including the binding and transport of heavy metal and lipophilic contaminants, fatty acids, amino acids, and thyroid hormones (Ganong 2001; Ucan-Marin et al. 2010). Albumins contain multiple sulfhydryl groups and thus can act as antioxidants, similar to circulating seleno-compounds, and limit toxicity by sequestration of Hg and other toxicants. Albumin has been reported to be a major binding protein for inorganic and organic forms of Hg in humans and mice (Lau and Sarka 1979; Yasutake et al 1989), and albumin-bound Hg has been proposed to be the primary mechanism for disposition and elimination of Hg via the kidney (Clarkson et al. 2007; Diamond and Zalups 1998; Khan and Wang 2010). The lower albumin concentrations in female polar bears compared to males, therefore, may contribute to a lower non-enzymatic antioxidant status of this cohort.
The significantly positive relationship between albumin and thyroxine, and the significantly negative relationship between albumin and PCBs, suggest that reductions in albumin may also contribute to a decreased binding capacity for the circulatory transport of thyroid hormones, especially in individuals also exposed to Hg. Thyroid hormone disruption in polar bears and other species has been hypothesized to involve the competitive binding of transthyretin by PCB metabolites in circulation (Braathen et al. 2004; Skaare et al. 2001). Concentrations of T4 and T3 released into the blood stream of mammals are transported bound to carrier proteins thyroxine-binding globulin, transthyretin, and albumin (Gagong 2001; Yamauchi and Ishihara 2009). These proteins maintain adequate concentration of thyroid hormones in plasma and their distribution to target cells (Gagong 2001). The relative proportions of these carrier proteins differ by species, season, and physiological state (Yamauchi and Ishihara 2009), and are unknown for polar bears. The circulating concentrations of ΣPCB7 of polar bears in the present study ranged from 2.03 to 132.8 ng/g ww. Svalbard polar bears having mean concentrations of 155 ng/g ww of PCBs in blood also had elevated concentration of hydroxy-metabolites of PCBs (e.g., 4-OH-CB107, 4-OH-CB146, and 4-OH-CB187) that saturated available transthyretin (Gutleb et al. 2010). Thyroid hormones that are not bound to transport proteins are more labile, more quickly degraded, and eliminated, which results in decreased circulating concentrations of hormones (Figure 9). PCB metabolites have also been proposed to increase the biliary elimination of hormones by the liver through the induction of biotransforming CYP-enzymes (Brouwer et al. 1998; McNabb and Fox 2003). Polar bears likely maintain thyroid homeostasis during periods of high toxicant exposure through release of negative feedback loops, and an increased production of T4 by the thyroid. Initial decreases in circulating concentrations of T4 (free and total fractions), therefore, can be compensated by a HPT level response (i.e., release of negative feedback to the pituitary) and an ultimate increased production of T4 by the thyroid gland. Activation of the HPT axis in response to subtle decreases in thyroid hormones has previously been described in vertebrates as evidenced by increased thyroid gland mass, alterations in thyroid gland histology, and elevated concentrations of thyroid hormones (Hall and Thomas 2007; Van Lente and Daher 1992; Webb and McNabb 2008). Positive correlations between contaminants and circulating concentrations of thyroid hormones have also been previously described in male polar bears (Sonne 2010), although the mechanisms driving these relationships are not currently understood. Our data suggest that as transthyretin becomes saturated by PCBs and their metabolites, elevations of T4 may involve binding with available albumin (Figure 9). Similar positive relationships between thyroid hormones and albumin have been described in euthyroid sick syndrome in humans (Van Lente and Daher 1992). HPT axis-level responses to different chemical mixtures, and the potential toxicological mechanisms driving these responses, have not been fully explored in polar bears and warrant further examination.
Figure 9.
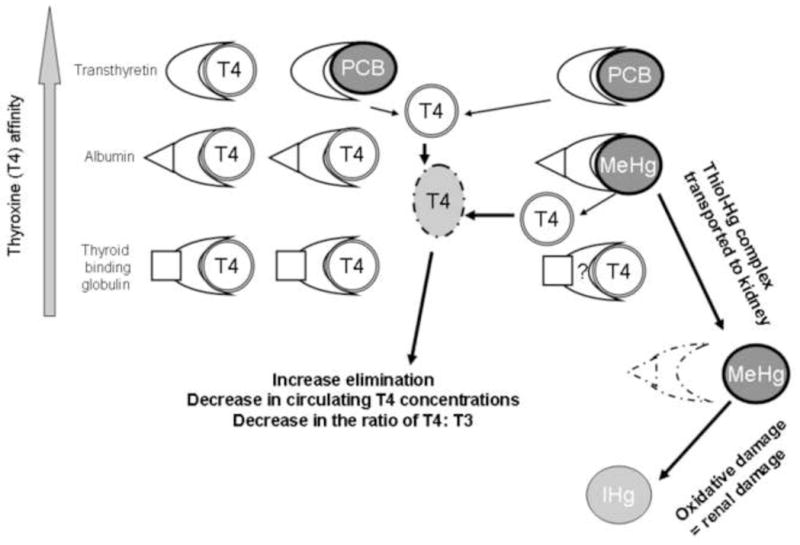
Conceptual model of the competitive displacement of thyroid hormone binding proteins (transthyretin, albumin, and thyroid binding globulin) by circulating toxicants.
As was expected, female polar bears had greater thyroid hormone concentrations (TT4, TT3, FT4, FT3) than males during spring. Greater thyroid hormone concentrations in females versus males are common among vertebrates, including polar bears, and suspected to relate to the regulation and interaction with estrogens (Braathen et al. 2004; Leatherland and Ronald 1981; Norris 2006; Skaare et al. 2001). Biological responses to toxicants in female polar bears would be expected to be more complex than males as females experience different physiologic and homeostatic states during spring such as recent fasting, lactation, caring for young, breeding activities, and estrous. These physiologic states are regulated by a combination of hormonal signals that include many of the biomarkers used in the assessment of toxic effects in polar bears. For example, thyroid hormones have reported to vary with lactation, pregnancy, molting, and fasting in vertebrates (Ahmed et al 2008; Hall and Thomas 2007; Hellgen 1998; Schussler and Orlando 1978). Thus, the examination of potential thyroid disruption by contaminants through analysis of circulating thyroid hormones alone may not accurately reflect an adverse biological effect in female polar bears. The negative correlation between toxicants and T4 in solitary females does not infer that this biological response is adverse, and correlation does not necessary reflect causation. Changes in thyroid status are tightly coupled to dose dependent changes in circulating toxicants and thus an acute biological response to toxicants can be compensated by HPT axis level responses (Figure 1). Our results suggest that multiple mechanisms including both toxic exposure and shifts in physiologic states (e.g., emergence from maternal denning, lactation, breeding activities) may be involved in the observed variations in thyroid status among polar bears. The use of blood-based biomarkers of thyroid status as indicators of adverse polar bear health are difficult to interpret without further information on thyroid gland morphology (e.g., status) or clinical signs of impairment that have not been identified in this sub-population of polar bears.
Although highly variable, the mean of total T4 concentration of polar bears in the present study (mean, 15 nmol/l) was three times lower than previously reported for presumably healthy captive and free-ranging polar bears (TT4, 50 nmol/l), although TT3 concentrations (present study, mean 1.4 nmol/l) were similar (TT3, 1.5 nmol/l; Churchill, Canada, Leatherland and Ronald 1981). Thyroid hormone concentrations of SBS polar bears were also lower than normal values for humans (TT4, 103 nmol/l; TT3, 2.3 nmol/l; Ganong 2001). Thyroid hormone concentrations of SBS polar bears were similar to those reported in Svalbard polar bears, but both populations also had similar concentrations of lipophilic toxicants (SBS polar bears in the present study, ΣPCB7, 2.03 to 132.8 ng/g ww; Svalbard bears, ΣPCB5, 16.7 to 203 ng/g ww; Braathen et al. 2004). TT3 concentrations for SBS polar bears were similar, but TT4 were lower, than female Greenland sledge dogs (Canis familiaris, TT3, 1.59 ± 0.12 nmol/l; TT4, 27.9 ± 1.65 nmol/l) exposed to an environmentally relevant concentration of contaminants (dietary PCBs, mean 2996 ng/g lw, Hg unreported; Sonne 2010). Congenital hypothyroid states have been reported in other ursids such as black bears (Ursus amerinanus) and associated with clinical signs including lethargy, inappetence, alopecia, and skeletal deformities (Duncan et al. 2002; Storms et al. 2004). The disruption of thyroid status has also been associated with the disturbance of neurotransmitters and antioxidant systems in the central nervous system in birds and mammals (Ahmed et al. 2008; McNabb and Fox 2003). Thyroid hormone associated effects in polar bears could not be determined in the present study, but a short-term disruption of thyroid hormone mediated activities in SBS polar bears is a plausible biological response to their present toxicant burden. Our data suggest that female and young polar bears are the cohorts of concern for chronic low-level exposure to chemical mixtures.
5. Conclusions
Circulating concentrations of Hg and ΣPCB7 were correlated in male and solitary female polar bears indicating concomitant exposure to heavy metal and lipophilic contaminants. In addition to dietary sources of contaminant exposure, the greater blood ΣPCB7 concentration of animals with low body condition scores suggests that mobilization of lipophilic contaminants from body storage sites to circulation was greater for females than males. Elevations of Se (whole blood and serum concentrations) with Hg suggest that seleno-compounds sequester and likely retain Hg compounds in circulation. Glutathione peroxidase activity increased with circulating concentrations of Hg suggesting that enzymatic antioxidants are also involved in the sequestration of Hg compounds and the reduction of free radicals. Polar bears likely maintain thyroid homeostasis during periods of toxicant exposure through HPT axis level responses and release of negative feedback loops, but this is uncertain. The use of blood-based biomarkers of selenium and thyroid status in polar bears for the assessment of adverse biological effects due to contaminants is not conclusive, especially in females that undergo changes in physiologic state throughout the year. Continued investigation of the physiologic states of polar bears, and potential clinical signs of impairment at the organ, individual, and population levels are needed to adequately address the biological impact of combined chemical exposures. A combination of studies using free-ranging and captive (zoo) bears, as well as histological assessments of tissues collected from animals obtained through legal subsistence hunts or found dead, could benefit these research efforts.
Highlights.
Se and TH biomarkers in blood were examined in polar bears
Biomarkers were compared to blood concentrations of Hg and ΣPCB7
Females were more susceptible to changes in Se and TH status than males
Positive and negative associations among biomarkers and contaminants are discussed
Further study of the possible adverse biological impact of combined toxicants is warranted
Acknowledgments
Funding for this project was provided by Grant Number 5P20RR016466 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. Laboratory analyses, staff support, and fellowship to K. Knott were provided by the Alaska IDEA Network of Biomedical Research Excellence (INBRE) Program. This research was also supported through a University of Alaska Fairbanks Center for Global Change Student Award funded by the Cooperative Institute for Alaska Research (CIFAR) with funds from the National Oceanic and Atmospheric Administration (NOAA) under cooperative agreement NA08OAR4320751 with the University of Alaska.
The project described was supported by Grant Number 5P20RR016466 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. Laboratory analyses, staff support, and fellowship to K. Knott were provided by the Alaska IDEA Network of Biomedical Research Excellence (INBRE) Program. This research was also supported through a UAF Center for Global Change Student Award funded by the Cooperative Institute for Alaska Research (CIFAR) with funds from the National Oceanic and Atmospheric Administration (NOAA) under cooperative agreement NA08OAR4320751 with the University of Alaska. We thank K. Alexander, S. Amstrup, J. M. Castellini, G. Durner, C. Ebner, C. Kirk, E. Regehr, K. Rode, K. Simac, R. Swor, and G. York for their excellent contributions in the field and laboratory. We are grateful for the contaminant and data analyses provided by B. Anulacion, R. Boyer, J. Bolton, J. Buzitis, R. Pearce, K. Tilbury and C. Sloan from NOAA Northwest Fisheries Science Center, and for thyroid analyses performed at the Diagnostic Center for Population and Animal Health at Michigan State University. We also thank L. Rea and K. Heuffer for their helpful comments on an earlier draft of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katrina K. Knott, Email: kkknott@alaska.edu.
Patricia Schenk, Email: schenk@dcpah.msu.edu.
Susan Beyerlein, Email: beyerlein@dcpah.msu.edu.
Daryle Boyd, Email: daryle.boyd@noaa.gov.
Gina M. Ylitalo, Email: gina.ylitalo@noaa.gov.
Todd. M. O’Hara, Email: tmohara@alaska.edu.
References
- Ahmed OM, El-Gareib AW, El-Bakry AM, El-Tawab SMA, Ahmed RG. Thyroid hormones states and brain development interactions. Int J Devel Neuro. 2008;26:147–209. doi: 10.1016/j.ijdevneu.2007.09.011. [DOI] [PubMed] [Google Scholar]
- AMAP. AMAP Assessment 2002: Heavy Metals in the Arctic. Arctic Monitoring and Assessment Programme (AMAP); Oslo, Norway: 2004a. p. 320. [Google Scholar]
- AMAP. Persistent Organic Pollutants (POPs) in the Arctic, Arctic Monitoring and Assessment Programme. AMAP; Oslo, Norway: 2004b. AMAP Assessment 2002; p. 320. [Google Scholar]
- Amstrup SC, Durner GM, Stirling I, Lunn NN, Messier F. Movements and distribution of polar bears in the Beaufort Sea. Can J Zool. 2000;78:948–966. [Google Scholar]
- Amstrup SC. Polar bear (Ursus maritimus) In: Feldhamer GA, Thompson BC, Chapman JA, editors. Wild Mammals of North America—Biology, Management, and Conservation. Baltimore: John Hopkins University Press; 2003. pp. 587–610. [Google Scholar]
- Atkinson SN, Ramsay MA. The effects of prolonged fasting of the body composition and reproductive success of female polar bears (Ursus maritimus) Funct Ecol. 1995;9:559–567. [Google Scholar]
- Atwell L, Hobson KA, Welch HE. Biomagnification and bioaccumulation of mercury in an arctic marine food web: insights from stable nitrogen isotope analysis. Can J Fish Aquat Sci. 1998;55:1114–1121. [Google Scholar]
- Bentzen TW, Follmann EH, Amstrup SC, York GS, Wooller MJ, Muir DCG, O’Hara TM. Dietary biomagnifications of organochlorine contaminants in Alaskan polar bears. Can J Zool. 2008a;86:177–191. [Google Scholar]
- Bernabucci U, Ronchi B, Lacetera N, Nardone A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J Dairy Sci. 2005;88:2017–2026. doi: 10.3168/jds.S0022-0302(05)72878-2. [DOI] [PubMed] [Google Scholar]
- Bernhoft A, Skaare JU, Wiig O, Derocher AE, Larsen HJS. Possible immunotoxic effects of organochlorines in polar bears (Ursus maritimus) at Svalbard. J Toxicol Env Heal A. 2000;59:561–574. doi: 10.1080/009841000156682. [DOI] [PubMed] [Google Scholar]
- Berry MJ, Ralston NVC. Mercury toxicity and the mitigating role of selenium. Ecohealth. 2008;5:456–459. doi: 10.1007/s10393-008-0204-y. [DOI] [PubMed] [Google Scholar]
- Braathen M, Derocher AE, Wiig O, Sormo EG, Lie E, Skaare JU, Jenssen BM. Relationships between PCBs and thyroid hormones and retinol in female and male polar bears. Environ Health Persp. 2004;112:826–833. doi: 10.1289/ehp.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radical Bio Med. 1999;27:951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Morse DC, Lans MC, Schuur AG, Murk AJ, Klasson-Wehler E, Bergman A, Visser TJ. Interactions of persistent environmental organohalogens with the thyroid hormone system: mechanisms and possible consequensces for animal and human health. Toxicol Indust Health. 1998;14:59–84. doi: 10.1177/074823379801400107. [DOI] [PubMed] [Google Scholar]
- Bruggeman V, Darras V. Dioxins, PCBs and iodine: effects on synthesis and metabolism of the iodine containing thyroid hormones. In: Preedy VR, Burrow GN, Watson R, editors. Comprehensive Handbook of Iodine: Nutritional, Biochemical, Pathological and Therapeutic Aspects. Academic Press; Burlington, Massechusettes: 2009. p. 1312. [Google Scholar]
- Burek KA, Gulland FMD, O’Hara TM. Effects of climate change on Arctic marine mammal health. Ecol Appl. 2008;18:S126–S134. doi: 10.1890/06-0553.1. [DOI] [PubMed] [Google Scholar]
- Cardona-Marek T, Knott KK, Meyer BE, O’Hara TM. Mercury concentrations in Southern Beaufort Sea polar bears: variation based on stable isotopes of carbon and nitrogen. Environ Toxicol Chem. 2009;28:1416–1424. doi: 10.1897/08-557.1. [DOI] [PubMed] [Google Scholar]
- Carmagnol F, Sinet PM, Jerome H. Selenium-dependent and non-selenium-dependent glutathione peroxidases in human-tissue extracts. Biochim Biophys Acta. 1983;759:49–57. doi: 10.1016/0304-4165(83)90188-5. [DOI] [PubMed] [Google Scholar]
- Cattet MRL, Caulkett NA, Obbard ME, Stenhouse GB. A body-condition index for ursids. Can J Zool. 2002;80:1156–1161. [Google Scholar]
- Chen CY, Yu HW, Zhao JJ, Li B, Qu LY, Liu SP, Zhang P, Chai Z. The roles of serum selenium and selenoproteins on mercury toxicity in environmental and occupational exposure. Environ Health Persp. 2006;114:297–301. doi: 10.1289/ehp.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Vyas JB, Ballatorl N. Mechanisms of mercury disposition in the body. Am J Ind Med. 2007;50:757–764. doi: 10.1002/ajim.20476. [DOI] [PubMed] [Google Scholar]
- Cunningham JG, Klein BG. Textbook of Veterinary Physiology. 4. Saunders; 2007. p. 700. [Google Scholar]
- Dehn LA, Sheffield GG, Follmann EH, Duffy LK, Thomas DL, Bratton GR, Taylor RJ, O’Hara TM. Trace elements in tissues of phocid seals harvested in the Alaskan and Canadian Arctic: influence of age and feeding ecology. Can J Zool. 2005;83:726–746. [Google Scholar]
- Derocher AE, Lunn NJ, Stirling I. Polar bears in a warming climate. Integr Comp Biol. 2004;44:163–176. doi: 10.1093/icb/44.2.163. [DOI] [PubMed] [Google Scholar]
- Diamond GL, Zalups RK. Understanding renal toxicity of heavy metals. Toxicol Pathol. 1998;26:92–103. doi: 10.1177/019262339802600111. [DOI] [PubMed] [Google Scholar]
- Dietz R, Riget F, Born EW. An assessment of selenium to mercury in Greenland marine animals. Sci Tot Environ. 2000;245:15–24. doi: 10.1016/s0048-9697(99)00430-1. [DOI] [PubMed] [Google Scholar]
- Duncan RB, Jones JCL, David H, Moon MM, Blodget D, Vaughan M. Cretinism in North American Black Bear (Ursus americanus) Vet Radiol Ultra. 2002;43:31–36. doi: 10.1111/j.1740-8261.2002.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Fairweather-Tait SJ, Collings R, Hurst R. Selenium bioavailability: current knowledge and future research requirements. Am J Clin Nutr. 2010;91:1484s–1491s. doi: 10.3945/ajcn.2010.28674J. [DOI] [PubMed] [Google Scholar]
- Fuglei E, Bustnes JO, Hop H, Mork T, Bjornfoth H, van Bael B. Environmental contaminants in arctic foxes (Alopex lagopus) in Svalbard: relationships with feeding ecology and body condition. Environ Poll. 2007;146:128–138. doi: 10.1016/j.envpol.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Ganong WF. Review of Medical Physiology. 20. The McGraw-Hill Companies, Inc; 2001. p. 870. [Google Scholar]
- Gutleb A, Cenijn P, Van Velzen M, Lie E, Ropstad E, Skaare JU, Malberg T, Bergman A, Gabrielsen GW, Legler J. In vitro assay shows that PCB metabolites completely saturate thyroid hormone transport capacity in blood of wild polar bears (Ursus maritimus) Environ Sci Technol. 2010;44:3149–3154. doi: 10.1021/es903029j. [DOI] [PubMed] [Google Scholar]
- Hellgren EC. Physiology of hibernation in bears. Ursus. 1998;10:467–477. [Google Scholar]
- Jenssen BM. Interacting effects of pollutants and climate on thyroid hormones in marine mammals. Mar Environ Res. 2006;62:S71–S71. [Google Scholar]
- Khan MAK, Wang F. Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. Environ Toxicol Chem. 2010;28:1567–1577. doi: 10.1897/08-375.1. [DOI] [PubMed] [Google Scholar]
- Kirk CM, Amstrup S, Swor R, Holcomb D, O’Hara TM. Hematology of Southern Beaufort Sea polar bears (2005–2007): biomarker for an arctic ecosystem health sentinel. EcoHealth. 2010 doi: 10.1007/s10393-010-0322-1. In press. [DOI] [PubMed] [Google Scholar]
- Klaassen CD. The Basic Science of Poisons. 6. 2001. Casarett and Doull’s Toxicology; p. 1236. [Google Scholar]
- Kohrle J, Jakob F, Contempre B, Dumont JE. Selenium, the thyroid, and the endocrine system. Endocrine Reviews. 2005;26:944–984. doi: 10.1210/er.2001-0034. [DOI] [PubMed] [Google Scholar]
- Kucklick JR, Struntz WDJ, Becker PR, York GW, O’Hara TM, Bohonowych JE. Persistent organochlorine pollutants in ringed seals and polar bears collected from northern Alaska. Sci Total Environ. 2002;287:45–59. doi: 10.1016/s0048-9697(01)00997-4. [DOI] [PubMed] [Google Scholar]
- Lau S, Sarka B. Inorganic mercury(II)-binding components in normal human blood serum. J Toxicol Environ Health. 1979;5(5):907–916. doi: 10.1080/15287397909529800. [DOI] [PubMed] [Google Scholar]
- Leatherland JF, Ronald K. Plasma concentrations of thyroid hormones in a captive and feral polar bear (Ursus maritimus) Comp Biochem Physiol. 1981;70A:575–577. [Google Scholar]
- Letcher RJ, Bustnes JO, Dietz R, Jenssen BM, Jorgensen EH, Sonne C, Verreault J, Vijayan MM, Gabrielsen GW. Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci Tot Environ. 2010;408:2995–3043. doi: 10.1016/j.scitotenv.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Mayne ST. Antioxidant nutrients and chronic disease: Use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr. 2003;133:933s–940s. doi: 10.1093/jn/133.3.933S. [DOI] [PubMed] [Google Scholar]
- McKinney MA, Peacock E, Letcher R. Sea ice-associated diet change increases the levels of chlorinated and brominated contaminants in polar bears. Environ Sci Technol. 2009;12:4334–4339. doi: 10.1021/es900471g. [DOI] [PubMed] [Google Scholar]
- McNabb FMA. Thyroid-hormones, their activation, degradation and effects on metabolism. J Nutr. 1995;125:S1773–S1776. doi: 10.1093/jn/125.suppl_6.1773S. [DOI] [PubMed] [Google Scholar]
- McNabb FM, Fox GA. Avian thyroid development in chemically contaminated environments: is there evidence of alterations in thyroid function and development? Evol Develop. 2003;5:76–82. doi: 10.1046/j.1525-142x.2003.03012.x. [DOI] [PubMed] [Google Scholar]
- Molner PK, Derocher AE, Thiemann GW, Lewis MA. Predicting survival, reproduction and abundance of polar bears under climate change. Biol Conserv. 2010;143:1612–1622. [Google Scholar]
- Newman MC. Fundamentals of Ecotoxicology. 3. CRC Press, Taylor and Francis Group; Boca Raton, FL: 2010. p. 541. [Google Scholar]
- Norris DO. Vertebrate Endocrinology. 4. Academic Press; 2006. p. 560. [Google Scholar]
- Polischuk SC, Norstrom RJ, Ramsay MA. Body burdens and tissue concentrations of organochlorines in polar bears (Ursus maritimus) vary during seasonal fasts. Environ Pollut. 2002;118:29–39. doi: 10.1016/s0269-7491(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Ralston NVC, Raymond LJ. Selenium’s protective effect against mercury toxicity. Neurotoxicology. 2006;27:1172–1172. [Google Scholar]
- Rode KD, Amstrup SC, Regehr EV. Reduced body size and cub recruitment in polar bears associated with sea ice decline. Ecol Applic. 2010;20:768–782. doi: 10.1890/08-1036.1. [DOI] [PubMed] [Google Scholar]
- Rolland RM. A review of chemically-induced alterations in thyroid and vitamin A status from field studies of wildlife and fish. J Wild Dis. 2000;36:615–635. doi: 10.7589/0090-3558-36.4.615. [DOI] [PubMed] [Google Scholar]
- Rosa C, O’Hara TM, Hoekstra PF, Refsal KR, Blake JE. Serum thyroid hormone concentrations and thyroid histomorphology as biomarkers in bowhead whales (Balaena mysticetus) Can J Zool. 2007;85:609–618. [Google Scholar]
- Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38:995–1014. doi: 10.1590/s0100-879x2005000700003. [DOI] [PubMed] [Google Scholar]
- Schussler GC, Orlando J. Fasting decreases triiodothyronine receptor capacity. Science. 1978;199:686–687. doi: 10.1126/science.204004. [DOI] [PubMed] [Google Scholar]
- Skaare JU, Bernhoft A, Wiig O, Norum KR, Haug E, Eide DM, Derocher AE. Relationships between plasma levels of organochlorines, retinol and thyroid hormones from polar bears (Ursus maritimus) at Svalbard. J Toxic Environ Health-Part A-Current Issues. 2001;62:227–241. doi: 10.1080/009841001459397. [DOI] [PubMed] [Google Scholar]
- Sloan CA, Brown DW, Pearce RW, Boyer RH, Bolton JL, Burrows DG, Herman DP, Krahn MM. Determining aromatic hydrocarbons and chlorinated hydrocarbons in sediments and tissues using accelerated solvent extraction and gas chromatography/mass spectrometry. In: Ostrander GK, editor. Techniques in Aquatic Toxicology. 2. CRC Press; Boca Raton, FL: 2005. [Google Scholar]
- Sonne C. Health effects from long-range transported contaminants in Arctic top predators: An integrated review based on studies of polar bears and relevant model species. Environ Intern. 2010;35:461–491. doi: 10.1016/j.envint.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Sonne C, Dietz R, Leifsson PS, Asmund G, Born EW, Kirkegaard M. Are liver and renal lesions in East Greenland polar bears (Ursus maritimus) associated with high mercury levels? Environ Health. 2007;6:11–20. doi: 10.1186/1476-069X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storms TN, Beazley SL, Schumacher J, Ramsay EC. Thyroid cystadenoma, colloid goiter, and hypthyroidism in an American black bear. J Zoo Wildlife Med. 2004;35:82–87. doi: 10.1638/03-033. [DOI] [PubMed] [Google Scholar]
- Tryland M, Brun E, Derocher AE, Arnemo JM, Kierulf P, Olberg RA, Wiig O. Plasma biochemical values from apparently healthy free-ranging polar bears from Svalbard. J Wild Dis. 2002;38:566–575. doi: 10.7589/0090-3558-38.3.566. [DOI] [PubMed] [Google Scholar]
- Tomasi TE, Hellgren EC, Tucker TJ. Thyroid hormone concentrations in black bears (Ursus americanus): hibernation and pregnancy effects. Gen Comp Endocr. 1998;109:192–199. doi: 10.1006/gcen.1997.7018. [DOI] [PubMed] [Google Scholar]
- Ucan-Marin F, Arukwe A, Mortensen AS, Gabrielsen GW, Letcher RJ. Recombinant albumin and transthyretin transport proteins from two gull species and human: chlorinated and brominated contaminant binding and thyroid hormones. Environ Sci Technol. 2010;44:497–504. doi: 10.1021/es902691u. [DOI] [PubMed] [Google Scholar]
- Van Lente F, Daher R. Plasma selenium concentrations in patients with euthyroid sick syndrome. Clin Chem. 1992;38:1885–1888. [PubMed] [Google Scholar]
- Wagemann R, Trebacz E, Boila G, Lockhart WL. Mercury species in the liver of ringed seals. Sci Total Environ. 2000;261:21–32. doi: 10.1016/s0048-9697(00)00592-1. [DOI] [PubMed] [Google Scholar]
- Webb CM, McNabb FMA. Polychlorinated biphenyl effects on avian hepatic enzyme induction and thyroid function. Gen Comp Endocrin. 2008;155:650–657. doi: 10.1016/j.ygcen.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Woshner V, Knott K, Wells R, Willetto C, Swor R, O’Hara T. Mercury and selenium in blood and epidermis of bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, FL: interaction and relevance to life history and hematologic parameters. Ecohealth. 2008;5:360–370. doi: 10.1007/s10393-008-0164-2. [DOI] [PubMed] [Google Scholar]
- Woshner VM, O’Hara TM, Bratton GR, Beasley VR. Concentrations and interactions of selected essential and non-essential elements in ringed seals and polar bears of arctic Alaska. J Wild Dis. 2001;37:771–721. doi: 10.7589/0090-3558-37.4.711. [DOI] [PubMed] [Google Scholar]
- Woshner VM, O’Hara TM, Eurell JA, Wallig MA, Bratton GR, Suydam RS. Distribution of inorganic mercury in liver and kidney of beluga and bowhead whales through autometallographic development of light microscopic tissue sections. Toxicol Pathol. 2002;30:209–215. doi: 10.1080/019262302753559542. [DOI] [PubMed] [Google Scholar]
- Yoneda S, Ohmichi M, Suzuki KT. Role of selenoprotein P in mechanisms of detoxification of inorganic mercury by selenium. Jpn J Tox Env Health. 1997;43:P26–P26. [Google Scholar]
- Yoneda S, Suzuki KT. Detoxification of mercury by selenium by binding of equimolar Hg-Se complex to a specific plasma protein. Toxicol Appl Pharm. 1997;143:274–280. doi: 10.1006/taap.1996.8095. [DOI] [PubMed] [Google Scholar]
- Yasutake A, Hiayama K, Inoue M. Mechanisms of urinary-excretion of methylmercury in mice. Archives Toxicol. 1989;63:479–483. doi: 10.1007/BF00316452. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Ishihara A. Evolutionary changes to transthyretin: developmentally regulated and tissue specific gene expression. Febs J. 2009;276:5357–5366. doi: 10.1111/j.1742-4658.2009.07245.x. [DOI] [PubMed] [Google Scholar]


