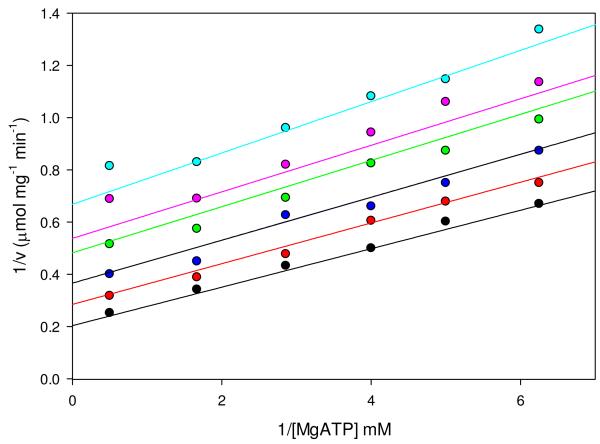
Figure 5.
Linear, noncompetitive inhibition of pyruvate carboxylation by phosphonoacetate with respect to MgATP. Initial rates were determined at varying concentrations of MgATP (0.16-2.0 mM) and fixed concentrations of phosphonoacetate (0 mM, black; 1.05 mM, red; 2.10 mM, blue; 3.6 mM, green; 4.2 mM, pink; 6.0 mM, cyan). Data were fitted to eqn (9) and the solid lines indicate the least-square fits to the equation. Kinetic parameters from these fits are shown in Table 8.