Figure 6.
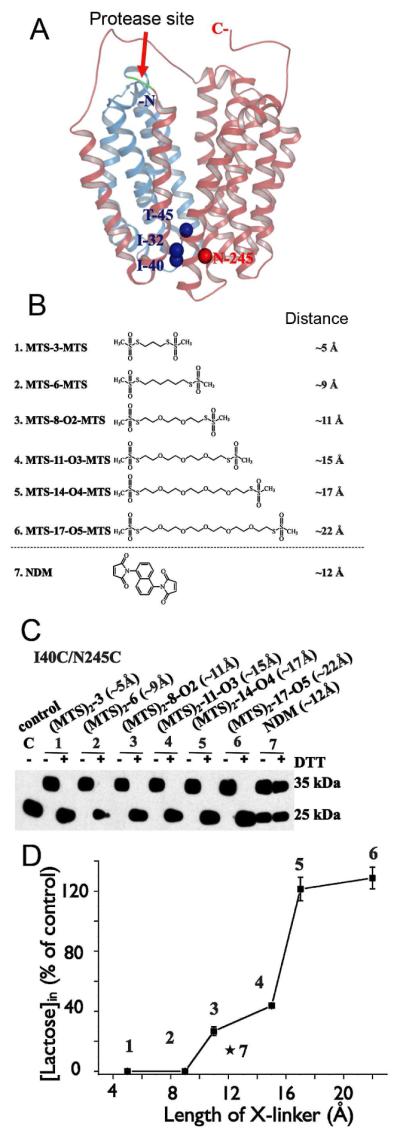
Effect of cross-linking at the periplasmic side of LacY on lactose transport. (A) Structural model of LacY with close periplasmic pathway. Cα atoms of the residues on periplasmic side used for Cys replacements are shown as blue (helices I and II) or red (helix VII) spheres. Red arrow indicates cleavage site for factor Xa protease located between helices IV and V. Helices I-IV and V-XII are colored in blue and pink, respectively. (B) Homo-bifunctional cross-linking reagents with approximate S-S distances between bridging sulfur atoms in the chains are shown. (C) Western blot analysis with anti-C-terminal antibody in cross-linking experiments after factor Xa protease digestion. Control – I40C/N245C mutant without addition of cross-linkers; 1 – 7, results of cross-linking with indicated reagents and effect of reducing agent (DTT). (D) Effect of cross-linking by different length reagents on lactose transport with mutant I40C/N245C. All experiments were performed with RSO vesicles.
