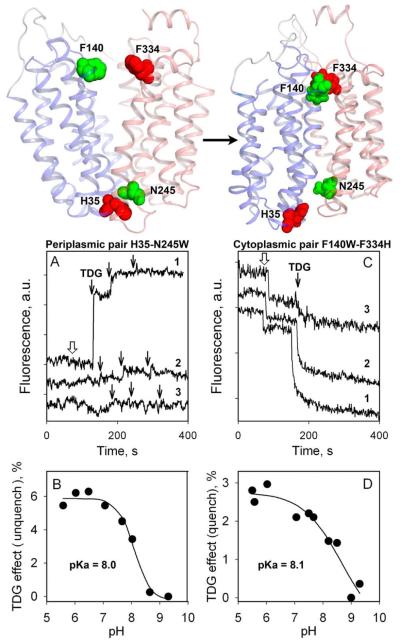
Figure 9.
Alternating access mechanism probed by quenching/unquenching of Trp fluorescence. (Top) Backbone of the inward-facing LacY structure (left) and outward facing model (right) with N-terminal and C-terminal 6-helix bundles colored in blue and pink, respectively. Arrow indicates the conformational change resulting from sugar binding. Residues used for Trp substitutions are shown as green spheres. Residues used as the quenchers of Trp fluorescence are presented as red spheres. (Bottom) Effect of conformational change in LacY triggered by sugar binding on Trp fluorescence. Excitation and emission wavelength are 295 and 330 nm, respectively. (A and B) Unquenching of Trp fluorescence in mutant N245W after addition of TDG. (C and D) Quenching of Trp fluorescence in mutant F140W/F334H after addition of TDG. (A) Trp fluorescence change after addition of sucrose (open arrow) or TDG (black arrows) to N245W mutant at pH 6 (trace 1), and pH 9 (trace 2), or to control LacY without Trp substitution at position 245 at pH 6 (trace 3). (B) Dependence of fluorescence change on pH for the N245W mutant. (C) Trp fluorescence change after addition of sucrose (open arrow) or TDG (black arrows) to F140W/F334W mutant at pH 5.5 (trace 1), pH 8.5 (trace 2), or pH 9.0 (trace 3). (D) Dependence of fluorescence change on pH for the F140W/F334W mutant.